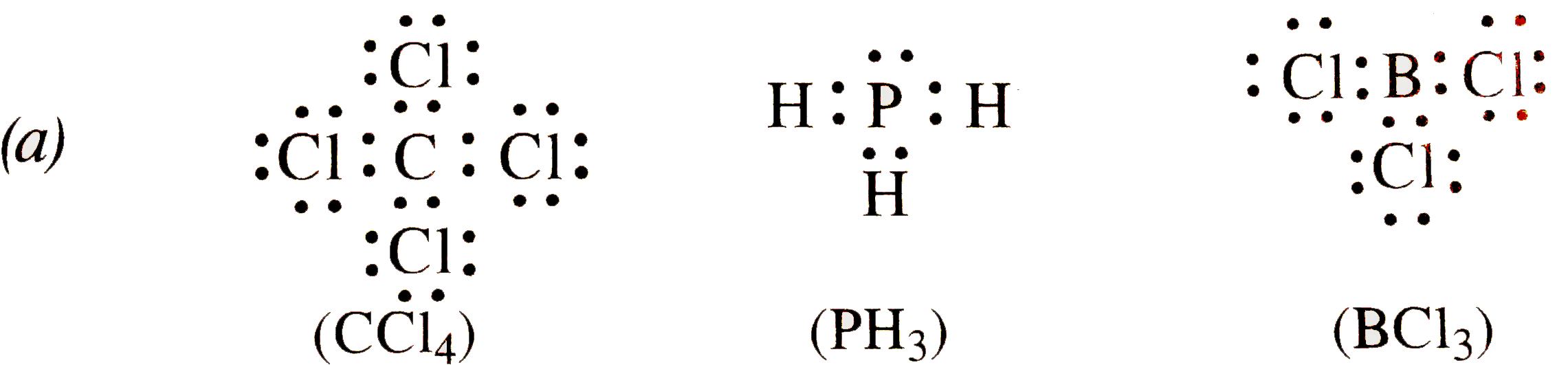Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise H.O.T.S. CONCEPTUAL QUESTIONS|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise VALUE BASED QUESTIONS|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise BORD EXAMINATIONS|84 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -ADDITIONAL IMPORTANT QUASTIONS
- Write the Lewis dot symbols of the following ions : Li^(+),Cl^(-),O^...
Text Solution
|
- Write the Lewis dot structures of (a) C Cl(4) (b) PH(3) ( c) BCl(3). I...
Text Solution
|
- Explain how valence bond theory accounts for (i) a carbon-carbn doub...
Text Solution
|
- Dary Lewis structures for H(2)CO(3),SF(6),PF(7)and CS(2). Is the octet...
Text Solution
|
- ELECTRONEGATIVITY & ELECTRON GAIN ENTHALPY
Text Solution
|
- Arrange the following in order of increasing ionic character : C-H,F...
Text Solution
|
- Which of the following has larger bond angle in the following pairs ? ...
Text Solution
|
- Out of intermolecular and intramolecular hydrogen bonding which has an...
Text Solution
|
- Discuss the shape of CO(2) molecule on the basis of hybridisation.
Text Solution
|
- Out of the following resonating strictures for CO(2) molecule, which a...
Text Solution
|
- Why is that in the SF(4) molecule, the lone pair of electrons occupies...
Text Solution
|
- Differentiate between VB theory and Lewis concept.
Text Solution
|
- Find out the number of sigma and pi bonds in the following molecules. ...
Text Solution
|
- Calculate the formula charge on each atom in a underset(* *)overset(* ...
Text Solution
|
- How many sigma and pi bonds are present in the molecules of toluene an...
Text Solution
|
- What is the hybridisatio carbon atoms numbered as 1,2 and 4 in the fol...
Text Solution
|
- What is meant by the term bond order ? Calculate the bond order ? Calc...
Text Solution
|
- The sdipole miment of hydrogen halides decreases form HF to HI. Explai...
Text Solution
|
- Which out of N(2) and H(2)O is polar and why ?
Text Solution
|
- Calculate the electronegativity value of chlorine on Mulliken's scale,...
Text Solution
|