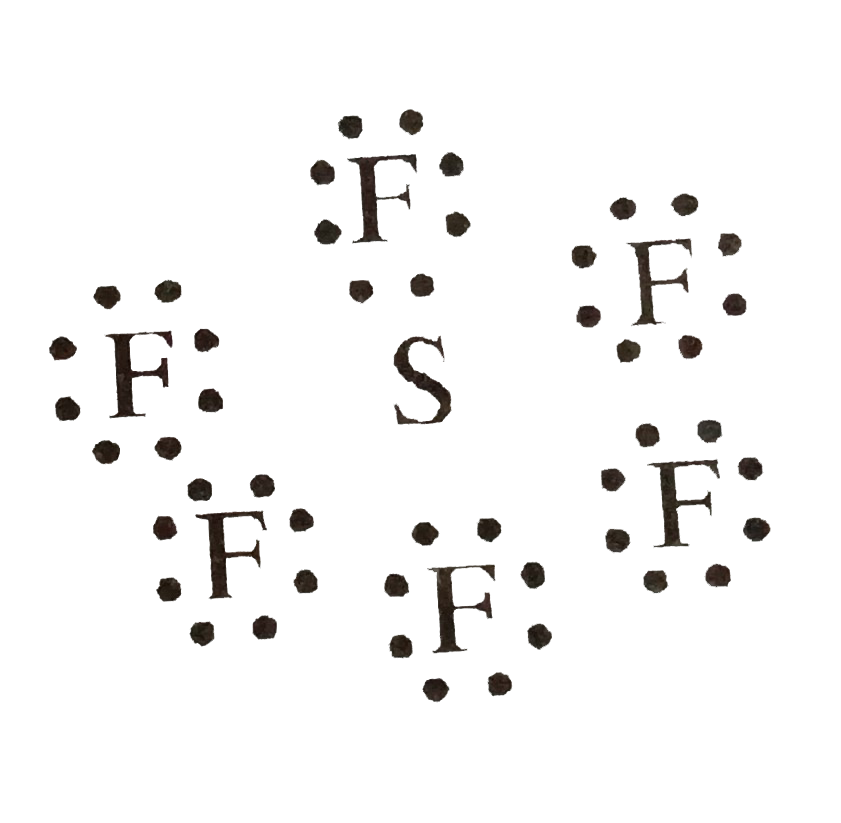Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise H.O.T.S. CONCEPTUAL QUESTIONS|11 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise VALUE BASED QUESTIONS|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise BORD EXAMINATIONS|84 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -ADDITIONAL IMPORTANT QUASTIONS
- (a) Which of the following species has greater polarising power ? (i...
Text Solution
|
- Why is lithium iodide more covalent than lithium fluoride ?
Text Solution
|
- Out of CS(2) and OCS which have higher dipole moment and why?
Text Solution
|
- Draw the Lewis structure of HCN.
Text Solution
|
- The presence of polar bonds in a polyatomic molecule suggests that the...
Text Solution
|
- Write two resonance structure of N2 O that satisfy the octet rule.
Text Solution
|
- Out of but-1-yne or but-1-ene which has higher dipole moment?
Text Solution
|
- Using VSEPR theory draw the shape of PCI(5) and BrF(5) ?.
Text Solution
|
- How does the bond length vary in dicarbon species C(2),C(2)^(-),C(2)...
Text Solution
|
- (a) How does bond energy vary from N(2)^(+) and N(2)^(-) and why ? ...
Text Solution
|
- Why is mobilty of H^(+) ions in ice greater as compared to liquid wate...
Text Solution
|
- According to Octed Rule, each atom gains or loses electrons to complet...
Text Solution
|
- L.C.A.O. principle is involved in the formation of molecular orbitals ...
Text Solution
|
- Arrange H(2)O,NH(3) and CH(4) molecules in decreasing order of bond an...
Text Solution
|
- Explain sp^(2) hybridisation by taking example of ethylene.
Text Solution
|
- In ionic solids, the oppositely charged ions are closely packed in spa...
Text Solution
|
- The study of dipole moment of a molecule is useful to explain the shap...
Text Solution
|
- Draw the structure and state polar and non-polar nature of (a) Ch(4) (...
Text Solution
|
- Drawn the molecular orbital diagram and write the bond order, magnetic...
Text Solution
|
- How do you differentiatie between sigma and pi bonds ?
Text Solution
|