A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Multiple Choice Question (Select the correct Answer)|13 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Assignment (Very Short Answer Question)|24 VideosSTATES OF MATTER : GASES AND LIQUIDS
DINESH PUBLICATION|Exercise Practice|40 VideosSTATES OF MATTER (SOLID STATE CHEMISTRY)
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|21 VideosSTRUCTURE OF ATOM
DINESH PUBLICATION|Exercise Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-Multiple Choice Question
- A person living in shimla observd that cooking without using pressure ...
Text Solution
|
- Which of the following property of water can be used to explain the sp...
Text Solution
|
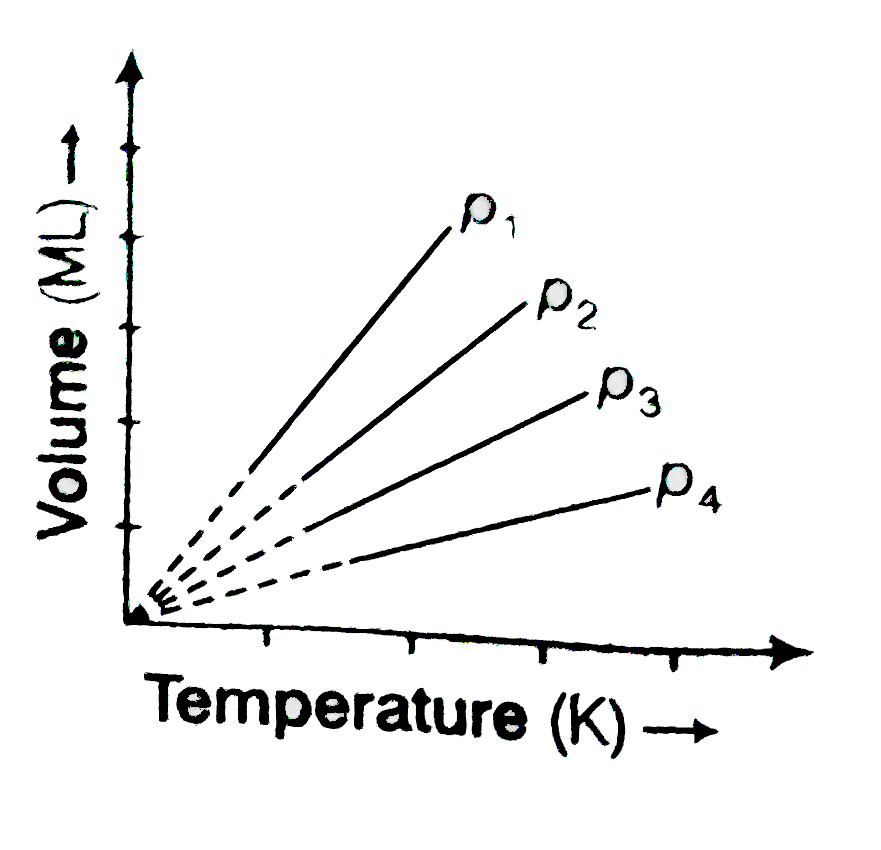
- A plot of volume (V) versus temperature (T) for a gas at constant pres...
Text Solution
|
- the interaction energy of London force is inversely proportional to si...
Text Solution
|
- Dipole-dipole forces act between the molecules possessing permanent di...
Text Solution
|
- the pressure of a 1 : 4 mixture of dihydrogen and dioxygen enclosed in...
Text Solution
|
- As the temperature increases, average kinetic energy of molecules incr...
Text Solution
|
- Gases posses characteristic critical temperature which depends upon th...
Text Solution
|
- What is SI unit of viscosity coefficient (eta) ?
Text Solution
|
- Atmospheric pressure recorded in different citie are as follows {:("...
Text Solution
|
- Which curve in figure represents the curve of ideal gas ?
Text Solution
|
- Increase in kinetic energy can overcome intermolecular forces of attra...
Text Solution
|
- How does the surface tension of a liquid vary with increase in tempera...
Text Solution
|
- With regard to the gaseous state of matter which of the following stat...
Text Solution
|
- Which of the following figures does not represent 1 mole of dioxygen g...
Text Solution
|
- Under which of the following conditions applied together, a gas deviat...
Text Solution
|
- Which of the following changes decrease the vapour pressure of water k...
Text Solution
|
- Match the graphs between the following variables with their names.
Text Solution
|
- Match the following gas laws with the equation representing them.
Text Solution
|
- Match the following graphs of ideal gas with their coordinates.
Text Solution
|