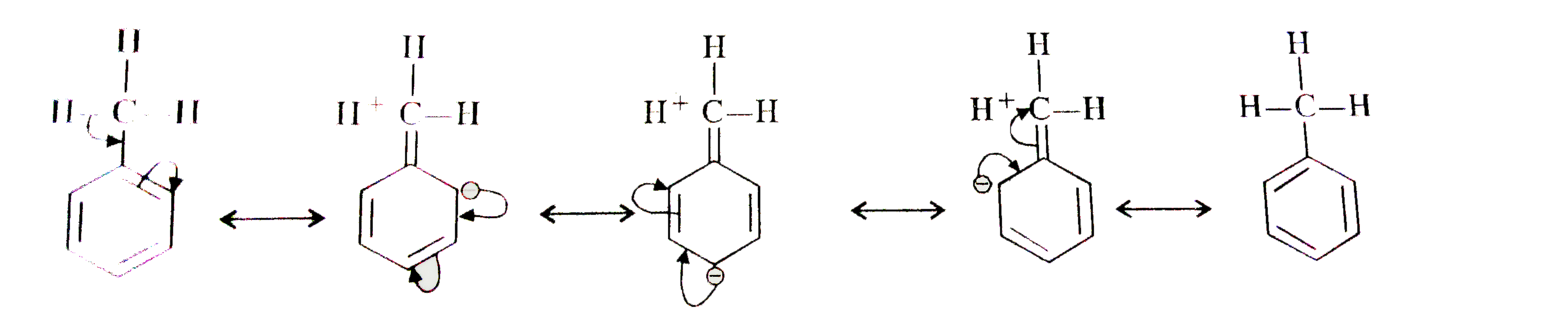
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise SHORT ANSWER TYPE QUESTION|33 VideosORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise CONCEPT BASED QUESTION|45 VideosORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES
DINESH PUBLICATION|Exercise PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUNDS|38 VideosNUCLEAR AND RADIO CHEMISTRY
DINESH PUBLICATION|Exercise Evaluate Yourself|45 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ORGANIC CHEMISTRY - SOME BASIC PRINCIPLES & TECHNIOUES-N.C.E.R.T.
- Which is expected to be more stable : O(2)NCH(2)CH(2)O^(-) or CH(3)CH(...
Text Solution
|
- Draw the resonance structures for the following compounds. Show the el...
Text Solution
|
- Explain why alkyl groups act as electron donors when attracted to a pi...
Text Solution
|
- What are nucleophiles and electrophiles. Explain with examples.
Text Solution
|
- Identify the reagents shown in bold in the following equations as nucl...
Text Solution
|
- Classify the following reactions in one of the reaction type studied i...
Text Solution
|
- What is the relation between the following pairs ? (a) (c) H-...
Text Solution
|
- Classify each of the following as homolysis as homolysis or heterolysi...
Text Solution
|
- Explain the terms inductive and electromeric effects. Which electron d...
Text Solution
|
- Give a brief description of the principle of the following processes t...
Text Solution
|
- Describe the method which can be used to separate two compounds with d...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|
- Describe the chemistry of Lassaigne's tets.
Text Solution
|
- Differentiate between the principle of estimation of nitrogen in an or...
Text Solution
|
- Describe the principle of estimation of halogens, sulphur and phosphor...
Text Solution
|
- Explain the principle of paper chromatography.
Text Solution
|
- Why is acid added to sodium extract before adding silver nitrate for t...
Text Solution
|
- Why is an organic compound fused with sodium for testing nitrogen, hal...
Text Solution
|
- Name a suitable technique of the components from a mixture of calcium ...
Text Solution
|
- An organic liquid vaporises at a temperature below its boiling point i...
Text Solution
|