Text Solution
Verified by Experts
Topper's Solved these Questions
DINESH PUBLICATION-ALDEHYDES AND KETONES -Interger
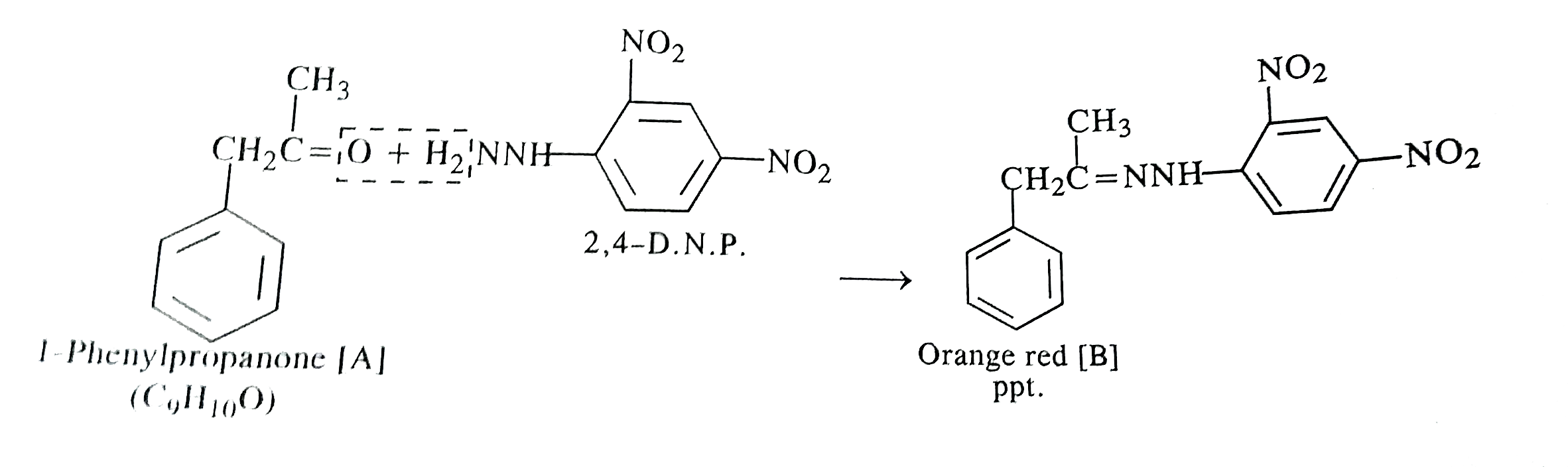
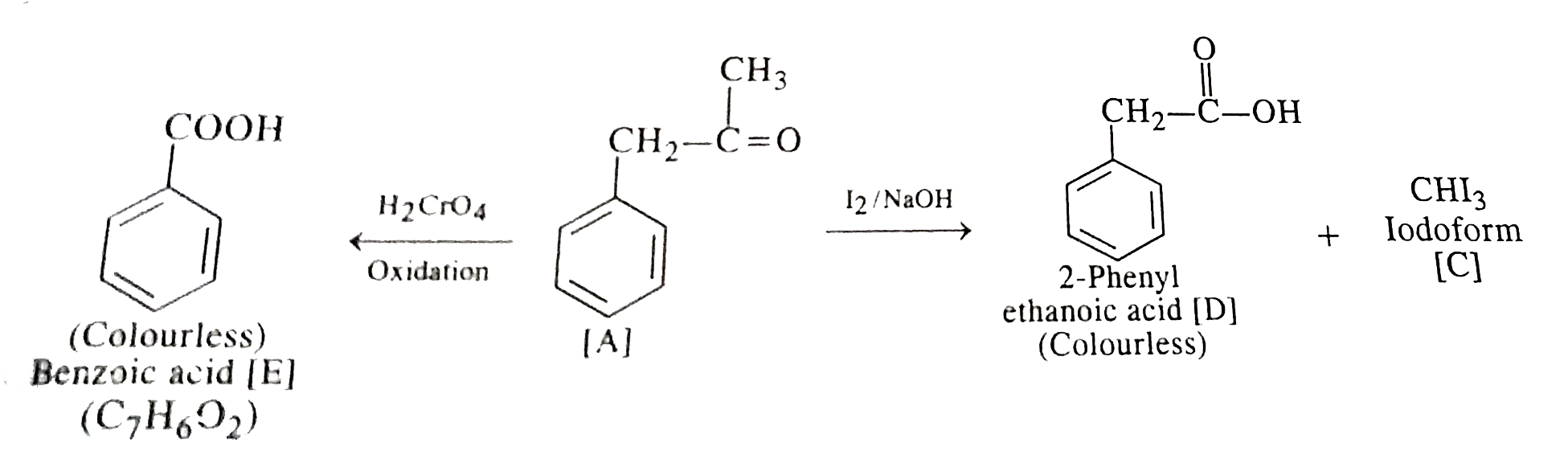
- An organic compound [A] with molecular formula C(9)H(10)O forms an ara...
Text Solution
|
- Total number of acyclic isomers having formula C(3)H(6)O :
Text Solution
|
- Isomeric aldehydes and ketones having the formula C(5)H(10)O are :
Text Solution
|
- 4.4g of CH(3)CHO upon oxidation with Tollen's reagenet form acid with ...
Text Solution
|
- In the compound CH(3)CH(2)COCH(3), the number of hydrogen atoms taking...
Text Solution
|
- Total number of nitrogen atoms present in urotropine is :
Text Solution
|