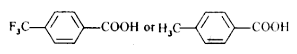Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise N.C.E.R.T. EXERCISE|19 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise SHORT ANSWER TYPE QUESTIONS|19 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise COMPLETION OF THE MISSING LINKS|1 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -N.C.E.R.T. IN-TEXT QUESTIONS
- Write the structures of the following compounds: (i) prop-Methoxypro...
Text Solution
|
- Write the structures of the products of the following reactions: ...
Text Solution
|
- Arrange the following compounds in increasing order of their boiling p...
Text Solution
|
- Arrange the following compounds in increasing order of their reactivit...
Text Solution
|
- Predict the products of the following reactions:
Text Solution
|
- Give the IUPAC names of the following compounds : (i) C(6)H(5)CH(2)C...
Text Solution
|
- Show how each of the following compounds can be converted to benzoic a...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|