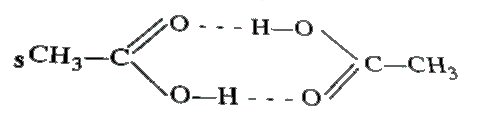
Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise QUESTIONS FROM BOARD EXAMINATIONS|67 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS (HOTS) QUESTIONS|7 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS|4 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -ADDITIONAL IMPORTANT QUESTIONS
- m-Hydroxybenzoic acid is a stronger acid than benzoic acid while p-hyd...
Text Solution
|
- Why does not formic acid form an anhydride upon heating?
Text Solution
|
- Completely anhydrous acetic has molecular mass of 120. Explain.
Text Solution
|
- Why is peroxy acid weaker than carboxylic acid ?
Text Solution
|
- Why is acetyl chloride a better acetylating agent than acetic acid ?
Text Solution
|
- Chloroformic acid does not exist while chloroethylformate exists. Expl...
Text Solution
|
- Carbon-oxygen bond lengths in formic acid are different but are the sa...
Text Solution
|
- Phenate ion has more number of contributing structures than benzoate i...
Text Solution
|
- Tertiary butyl benzene does not give benzoic acid when oxidised with K...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- CH(3)COO^(-) ion is more stable than C(2)H(5)O^(-) ion. Assign reason.
Text Solution
|
- Peroxyacetic acid is a weaker acid than acetic acid. Explain.
Text Solution
|
- Why are boiling of aldehydes and ketones lower than those of the corre...
Text Solution
|
- Which out of each pair is expected to be a stornger acid ? (a) CH(3)...
Text Solution
|
- What is glacial acetic acid ? Why is it so named ?
Text Solution
|
- How will you prepare 2-methylbutanoic acid from butan-2-01 ?
Text Solution
|
- How will you convert propionic acid to acetic acid ?
Text Solution
|
- How will you prepare butanoic acid from 1-bromopropane?
Text Solution
|
- Suggest an oxidising agent to convert (CH(3))(2)C=CHCOCH(3)" to "(CH(3...
Text Solution
|
- Explain Why : (i) Carboxylic acids are stronger acids than alcohols....
Text Solution
|