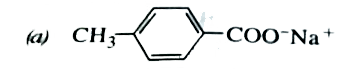
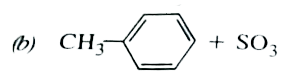
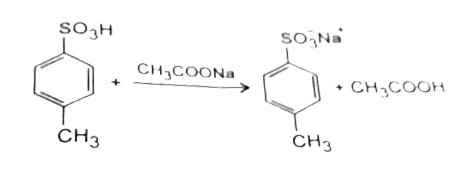
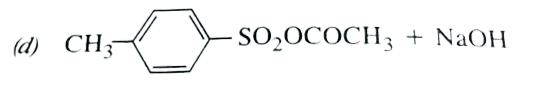A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise MATRIX-MATCH TYPE QUESTIONS|5 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise ASSIGNMENT|56 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -MULTIPLE CHOICE QUESTION BANK (MCQB)
- The acidity of carboxylic acid is determined by the nature of the alky...
Text Solution
|
- The acidity of carboxylic acid is determined by the nature of the alky...
Text Solution
|
- The acidity of carboxylic acid is determined by the nature of the alky...
Text Solution
|
- Chlorination of toluene in the presence of light and heat followed by ...
Text Solution
|
- Identify the final product of the following reaction :
Text Solution
|
- Consider the following reaction underset("Ester(x)")underset()(C(4)H...
Text Solution
|
- Which of the following carboxylic acids undergoes decarboxylation easi...
Text Solution
|
- In the reaction C(6)H(5)CO OCH(3)overset(LiAlH(4))to? the products f...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- Which of the following compounds is most susceptible to a nucleophilic...
Text Solution
|
- When propionic acid is treated with aqueous sodium bicarbonate, CO(2) ...
Text Solution
|
- Ethyl easter overset(CH(3)MgBr)underset("excess")rarrP, the product P ...
Text Solution
|
- When benzamide is treated with POCl(3) the product is
Text Solution
|
- When reacts with CH(3)COO^(-)Na^(+) (excess), the
Text Solution
|
- The correct order of acidity of the following is
Text Solution
|
- The carboxyl functional group (-COOH) is present in :
Text Solution
|
- The compound that undergoes decarboxylation most readily under mild c...
Text Solution
|
- The major product(s) of the following reaction is (are)
Text Solution
|
- P and Q are isomers of dicarboxylic acid C(4)H(4)O(4). Bothdecolorize ...
Text Solution
|
- Among the following compounds, the one (s) that gives (gives) efferves...
Text Solution
|