A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise MATRIX-MATCH TYPE QUESTIONS|5 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)|13 VideosCARBOXYLIC ACIDS
DINESH PUBLICATION|Exercise ASSIGNMENT|56 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise Unit Test - 1|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CARBOXYLIC ACIDS -MULTIPLE CHOICE QUESTION BANK (MCQB)
- The compound that undergoes decarboxylation most readily under mild c...
Text Solution
|
- The major product(s) of the following reaction is (are)
Text Solution
|
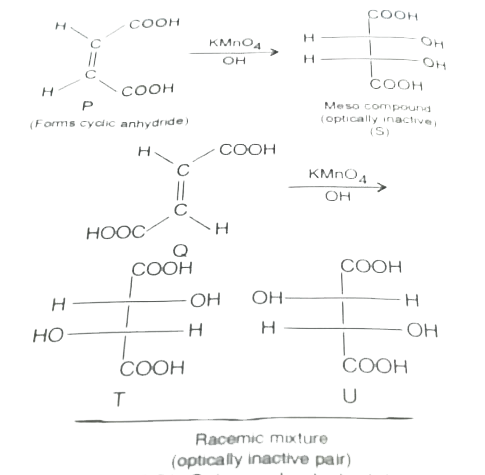
- P and Q are isomers of dicarboxylic acid C(4)H(4)O(4). Bothdecolorize ...
Text Solution
|
- Among the following compounds, the one (s) that gives (gives) efferves...
Text Solution
|
- The correct order of acidity for the following compounds is
Text Solution
|
- The acids which do not contain a-COOH group are :
Text Solution
|
- Phenol and benzoic acid may be distinguished by their reaction with :
Text Solution
|
- Which of the following on oxidation with alkaline KMnO(4) followed by ...
Text Solution
|
- Which of the following statements is/are correct about formic acid?
Text Solution
|
- Assertion : Benzoic acid is a weaker acid than formic acid. Reason :...
Text Solution
|
- Acetic acid does not undergo haloform reaction. Acetic acid has no a...
Text Solution
|
- Assertion: Formic acid reduces mercuric chloride to mercurous chloride...
Text Solution
|
- Assertion : Nitration of benzoic acid gives meta nitrobenzoic acid. ...
Text Solution
|
- Assertion : Carbxoylic acids donot give characteristic reactions of ca...
Text Solution
|
- Assertion : Upon heating an amide with Br(2)" and "KOH, primary amine ...
Text Solution
|
- Assertion : Carboxylic acids are stronger acids than phenols. Reason...
Text Solution
|
- Assertion : Although fluorine is more electronegative than chlorine, p...
Text Solution
|
- Assertion : Acid amides are weakly basic in nature. Reason : Basic c...
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol. R...
Text Solution
|
- Assertion: Formic acid reduces mercuric chloride to mercurous chloride...
Text Solution
|
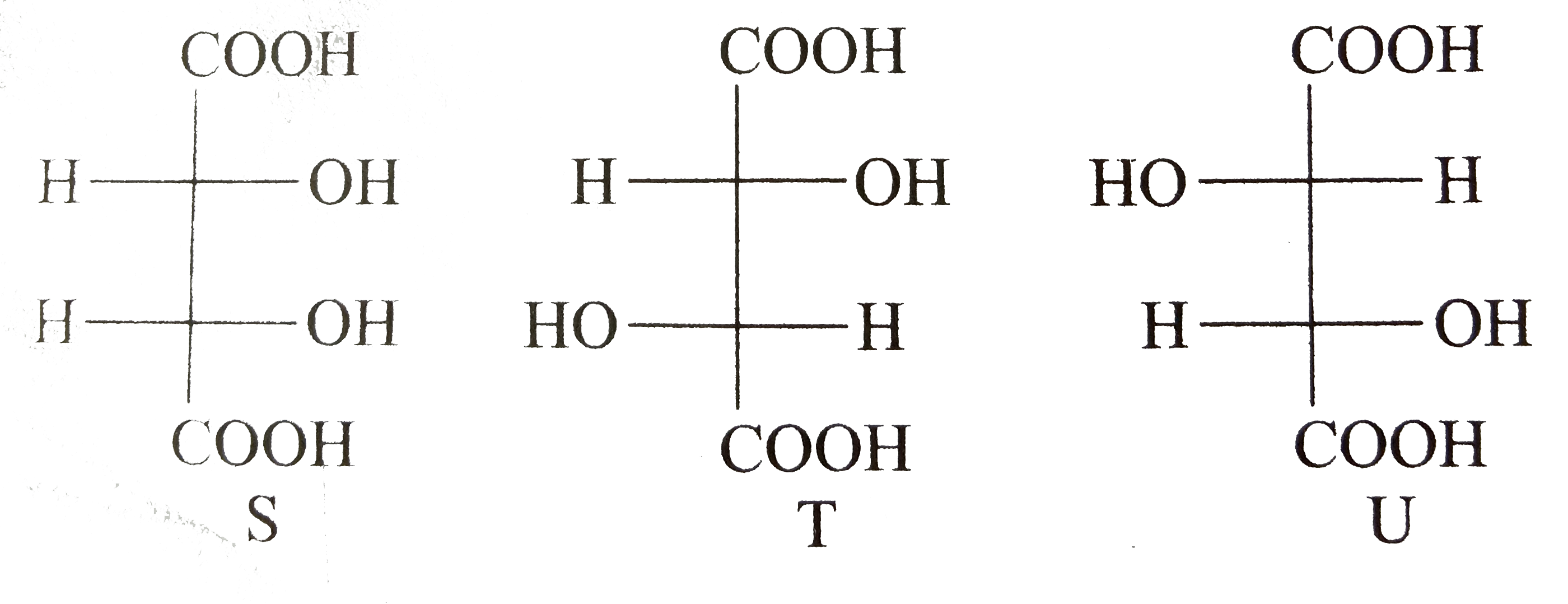 Compounds formed from `P` and `Q` are, respectively
Compounds formed from `P` and `Q` are, respectively