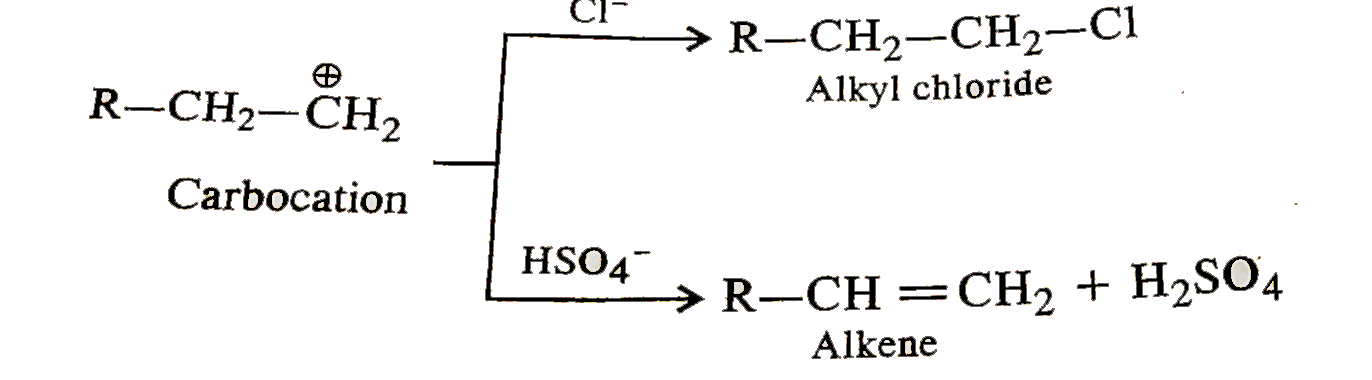
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise QUESTION FROM BOARD EXAMINATION|77 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS|8 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise NCERT Exercise|23 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Interger|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-ADDITIONAL IMPORTANT QUESTION
- Alcohols react with halogen acids as well as phosphorus halides to for...
Text Solution
|
- The order of reactivity of alcohols in the esterification reaction is ...
Text Solution
|
- Dehydration of alcohol to form an alkene is always carried out with co...
Text Solution
|
- Why is phenol acidic and hexanol neutral towards solution of NaOH ?
Text Solution
|
- Which of the following is the most reactive towards attack by an elect...
Text Solution
|
- Why is cyclohexanol more soluble in water than hexan-1-ol ?
Text Solution
|
- How will you distinguish between CH(3)(CH(2))(3)OH and CH(3)CH=CHCH(2)...
Text Solution
|
- Why can not anhydrous calcium chloride be used for drying ethyl alcoho...
Text Solution
|
- What happens when propan-1-ol is treated with ethanoic acid in the pre...
Text Solution
|
- How will you distinguish between allyl alcohom and n-propyl alcohol ?
Text Solution
|
- Give the product of reaction of ethyl alcohol with conc. H(2)SO(4) at ...
Text Solution
|
- Give a simple chemical test to distinguish between : (i) Ethanol and...
Text Solution
|
- Give a chemical test to distinguish between methanol and ethanol.
Text Solution
|
- Arrange the following in order of decreasing boiling points (i) Pent...
Text Solution
|
- Predict in which of the following cases, the reaction with Lucas reage...
Text Solution
|
- Outline the synthesis of the following alcohols from the indicated sta...
Text Solution
|
- Compare the relative acidic strength of the following: (i) CH(3)OH (...
Text Solution
|
- What is the difference in the nature of alcohol when propene is subjec...
Text Solution
|
- Give the IUPAC names for each of the following compounds.
Text Solution
|
- Write the number of resonating structures that can be written for the ...
Text Solution
|