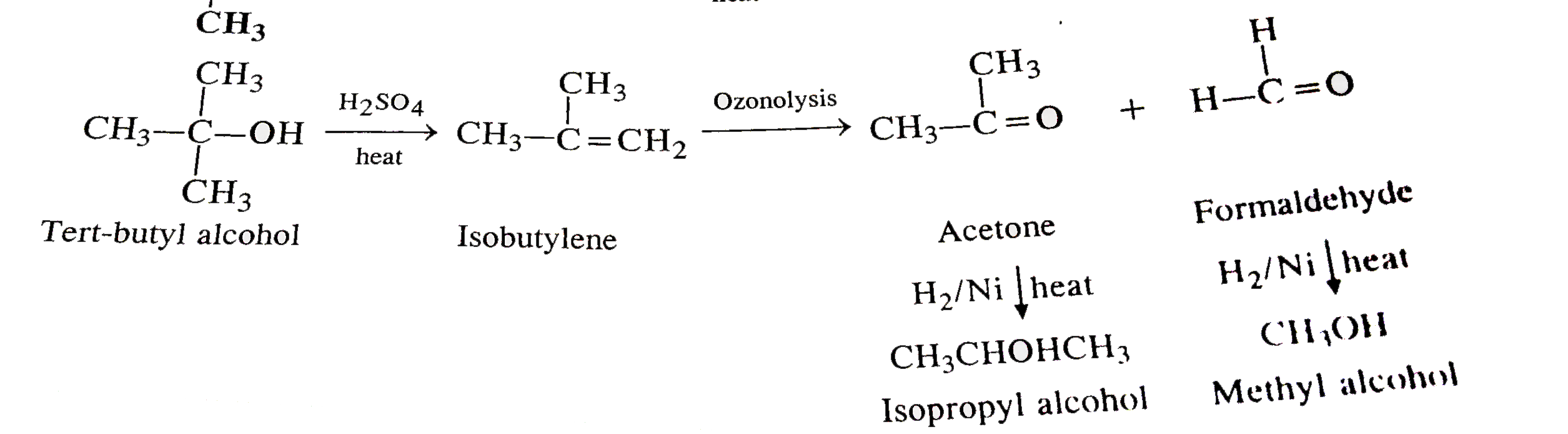Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS|8 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise PROBLEM|9 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTION|30 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Interger|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-QUESTION FROM BOARD EXAMINATION
- How will you convert propanone to tertiary butyl alcohol ?
Text Solution
|
- Complete the following :
Text Solution
|
- Complete the following chemical reactions CH(3)-underset(CH(3))under...
Text Solution
|
- Describe a chemical test to distinguish between the following pairs : ...
Text Solution
|
- Give the IUPAC name of the compound : CH(3)-underset(CH(3))underset(|)...
Text Solution
|
- Give the IUPAC name of the compound : CH(2)=CH-underset(OH)underset(|)...
Text Solution
|
- Why is phenol more acidic than ethanol ?
Text Solution
|
- How will you convert phenol to benzoquinone ?
Text Solution
|
- Name the reagents which can be used for the following conversions : ...
Text Solution
|
- o- and p- nitrophenols are stronger acids than phenol. Explain.
Text Solution
|
- Write structure of phenyl isopentyl ether. Give its IUPAC name.
Text Solution
|
- Give chemical tests to distinguish between (i) Isopropyl alcohol and...
Text Solution
|
- Give possible explanation for the following. (a) Ortho-nitrophenol i...
Text Solution
|
- (a) Give the mechanism of the following reaction : 2CH(3)CH(2)OH und...
Text Solution
|
- How would you obtain 2-Methylpropan-2-ol from Methyl magnesium bromide...
Text Solution
|
- Explain the following with an example: i. Kolbe's reaction ii. R...
Text Solution
|
- Give the IUPAC names of : (i) CH(3)O-CH(2)-CH(2)-OCH(3) (ii) CH(3)-u...
Text Solution
|
- Draw the structure and the name of the product when the following alco...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- An alkoxide ion is a stronger base than hydroxide ion. Justify.
Text Solution
|