Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS|8 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise PROBLEM|9 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTION|30 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Interger|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-QUESTION FROM BOARD EXAMINATION
- Draw the structure and the name of the product when the following alco...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- An alkoxide ion is a stronger base than hydroxide ion. Justify.
Text Solution
|
- Write the mechanism for the preparation of ethanol from ethane.
Text Solution
|
- How will you convert methanol into ethanoic acid ?
Text Solution
|
- CH(3)CH(2)OH underset(443 K)overset(Conc. H(2)SO(4))(rarr) X+H(2)O C...
Text Solution
|
- How will you convert phenol to 2, 4, 6-trinitrophenol ?
Text Solution
|
- Arrange the following compounds in increasing of order of their acidic...
Text Solution
|
- How will you distinguish between benzyl alcohol and phenol ?
Text Solution
|
- Explain esterification reaction.
Text Solution
|
- Explain mechanism of dehysration of alcohols to give alkenes.
Text Solution
|
- Write any teo difference between methyl alcohol and ethyl alcohol.
Text Solution
|
- o- nitrophenol is steam volatile while p-nitrophenol is not. Discuss.
Text Solution
|
- Give the structures of A, B and C in the following reaction : CH(3)B...
Text Solution
|
- Name the reagents used in the following reactions: i. Oxidation of ...
Text Solution
|
- How will you prepare benzene from phenol ?
Text Solution
|
- How will you explain that phenols are acidic in nature ?
Text Solution
|
- How will you prepare alcohols from alkyl halides and alkenes ? Write c...
Text Solution
|
- Which compound is formed when a secondary alcohol is oxidized ?
Text Solution
|
- Write the chemical reaction of enthanol with PCl(5) and PCl(3) separat...
Text Solution
|
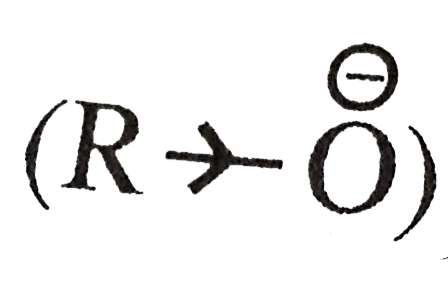 , electron density on the alkoxide ion is moreas compared to hydroxide ion `(OH^(Θ))`. It can therefore, accept a `H^(+)` more easily and is a stronger base than the hydroxide ion.
, electron density on the alkoxide ion is moreas compared to hydroxide ion `(OH^(Θ))`. It can therefore, accept a `H^(+)` more easily and is a stronger base than the hydroxide ion.