Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-PROBLEM
- [A] underset(250^(@)C)overset(Al(2)O(3))(rarr) [B] underset((ii) AgOH)...
Text Solution
|
- Identify A to D in the following reactions : [D] underset(H(3)O^(+))...
Text Solution
|
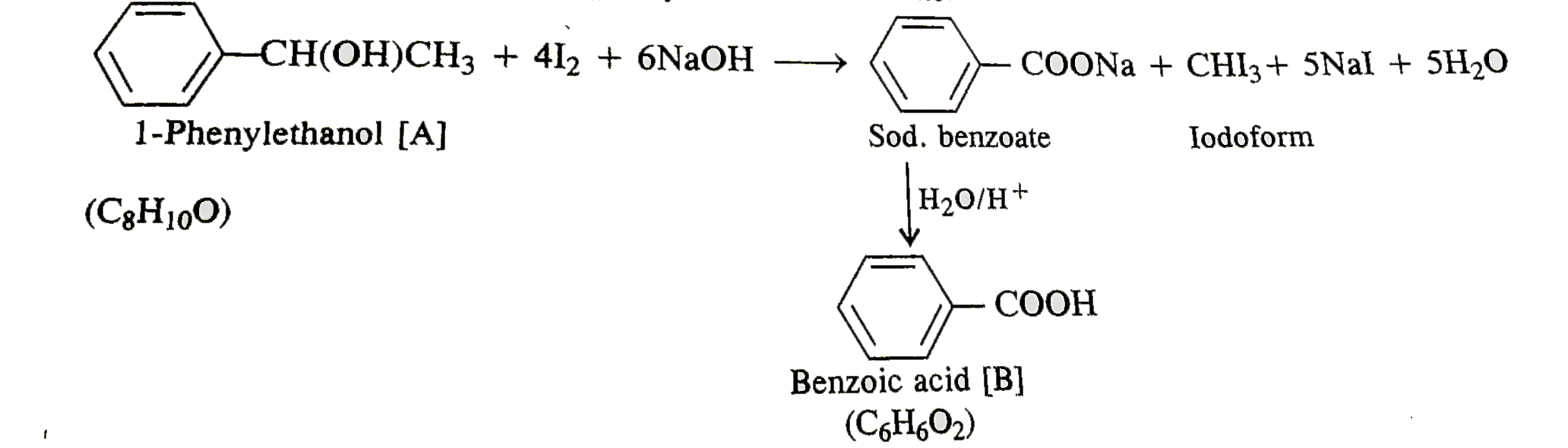
- A compound A (C(8)H(10)O) upon treatment with alkaline solution of iod...
Text Solution
|
- Identify A, B, C and D in the following :
Text Solution
|
- An organic compound (A) has 76.6% C, 6.38% H. Its vapour density is 47...
Text Solution
|
- The acid catalysed hydration of the compound A produces the compound B...
Text Solution
|
- An organic compound 'A' having molecular formula C(6)H(6)O gives a cha...
Text Solution
|
- A compound [A] with molecular formula C(4)H(10)O reacts rapidly with m...
Text Solution
|
- An alcohol [A] with molecules formula (C(4)H(10)O) o oxidation with ac...
Text Solution
|