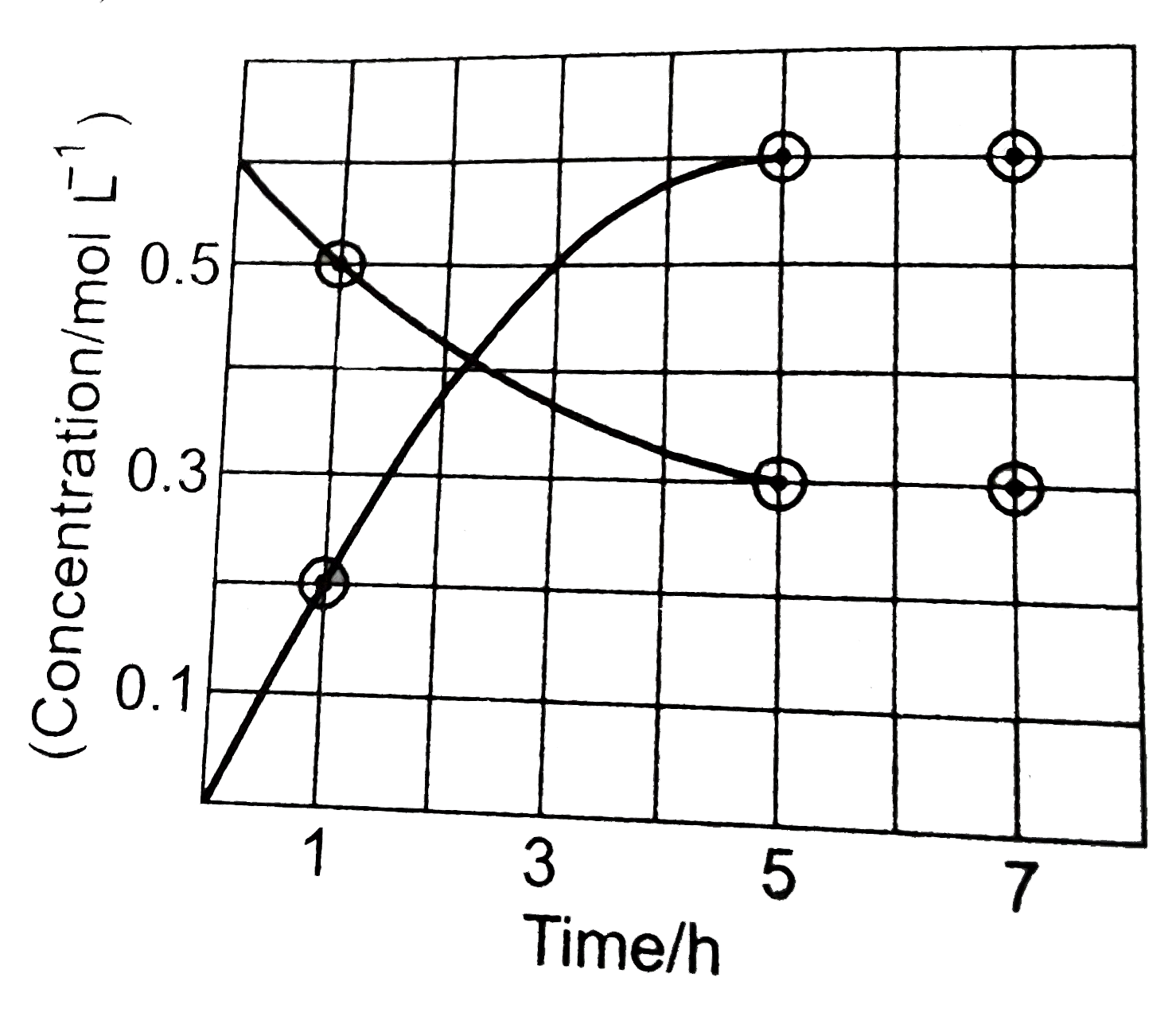
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Matrix Match|2 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Integer|4 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Selected Straight|49 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Reasoning Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-RATES OF REACTIONS AND CHEMICAL KINETICS-Comprehension
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The energy profile diagrams are very important tool through which, we ...
Text Solution
|
- The process of the reaction A hArr nB with time is represented in the ...
Text Solution
|
- The progress of the reaction A hArr nB with time is presented in the f...
Text Solution
|
- The progress of reaction AhArrnB with time, is represented in fig ...
Text Solution
|