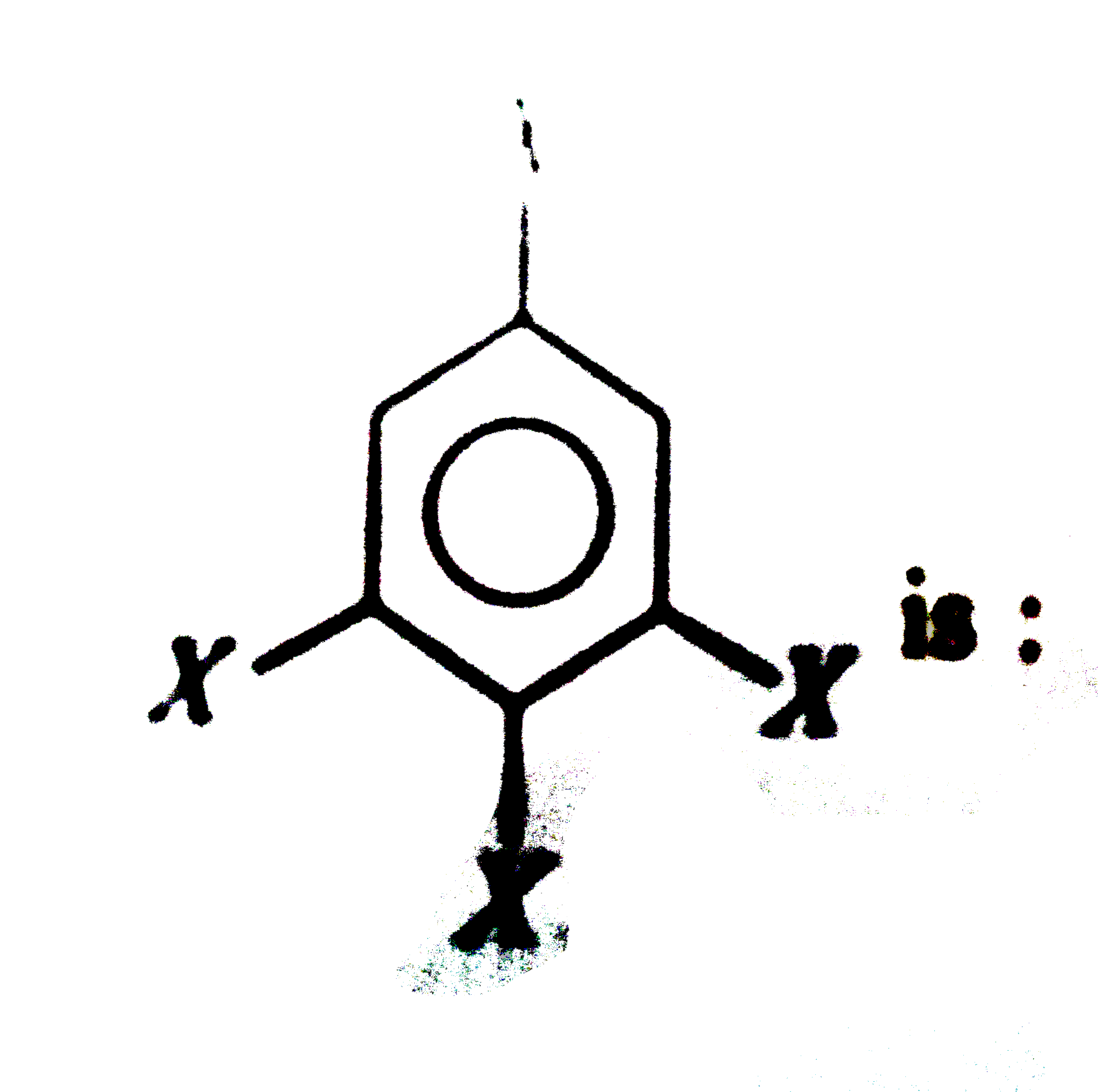A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-INORGANIC CHEMISTRY
- Which of the following represent most effective pi-bond
Text Solution
|
- In which reaction hybridisation of underlined atom does not change.
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- Which of the following compound has non-zero dipole moment
Text Solution
|
- Which of the following molecule is planar due to back bonding
Text Solution
|
- Amongst the following , molecule having maximum bond angles of 90^(@) ...
Text Solution
|
- Which of the following statement is incorrect
Text Solution
|
- The coordinate bond is absent in
Text Solution
|
- Which of the following is least stable
Text Solution
|
- The true statement from the following are
Text Solution
|
- The bond order for NO and NO^(+), respectively are
Text Solution
|
- Back bonding always changes
Text Solution
|
- Bond angle in H(2)O is
Text Solution
|
- The correct stability order for N2 and its given ions is :
Text Solution
|
- Which of the following have same bond order :- (I) CO (II)CN^(-) (II...
Text Solution
|
- In XeF(2),XeF(4)" and "XeF(6), the number of the lone pairs of Xe resp...
Text Solution
|
- Which of the following hydroxides is the most soluble in water ?
Text Solution
|
- The correct order of N-O bond length is
Text Solution
|
- The correct order of A-O-A bond angle of (A=H,F or Cl)
Text Solution
|
- There are some species given below :- {:((a)O(2)^(+),,,,(b)CO),((c)B...
Text Solution
|
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of