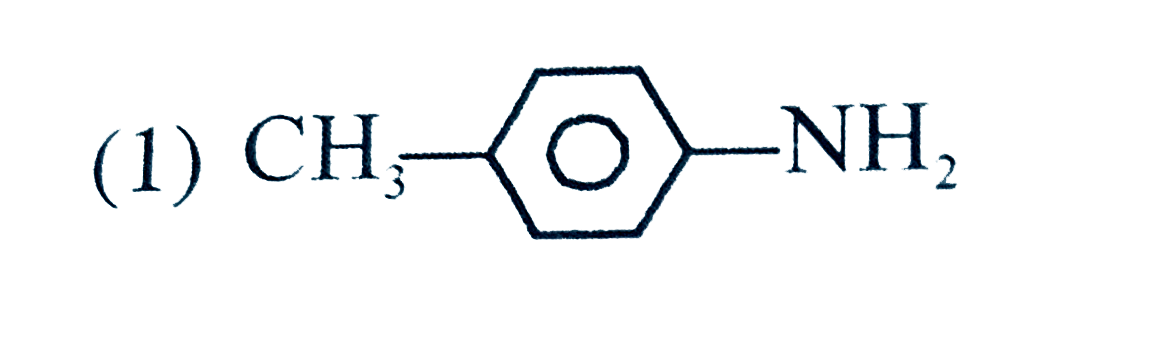
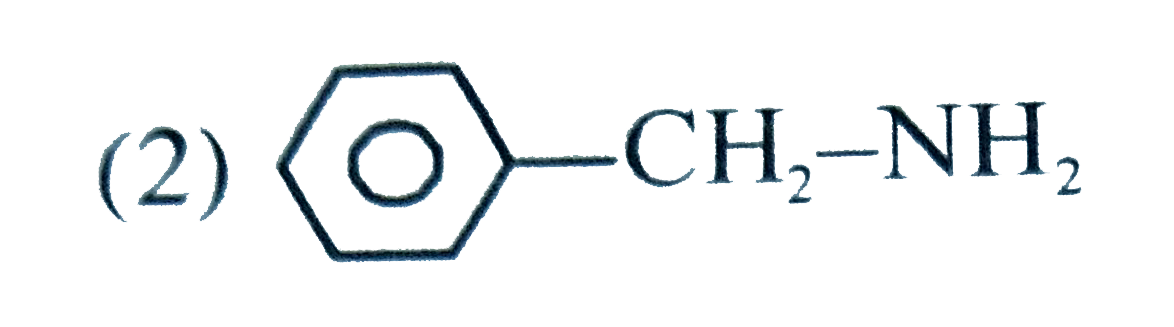
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-CHEMISTRY AT A GLANCE-ORGANIC CHEMISTRY
- Arrange the following in decreasing order of boiling point : (I) CH...
Text Solution
|
- An compound react with Hinsberg's reagent gives a product which is sol...
Text Solution
|
- Which of the following compound cannot be formed by Gabriel phthalimid...
Text Solution
|
- Find out major product
Text Solution
|
- Product ( C) and (D ) will be:
Text Solution
|
- Product C and D are respectively:
Text Solution
|
- Which of the following conversion is incorrect :
Text Solution
|
- Which of the following compound gives yellow oily liquid nitrosamine w...
Text Solution
|
- Aniline can be obtained by : (a) C(6)H(5)COOHunderset(H^(o+))overse...
Text Solution
|
- Aniline cannot be obtained by :
Text Solution
|
- Aline can be prepares by :
Text Solution
|
- Compound A is :
Text Solution
|
- Compound B is :
Text Solution
|
- Find out (A) :
Text Solution
|
- Product (A) is :
Text Solution
|
- The reagents needed to convert :
Text Solution
|
- Aniline underset(o-5^(@)C)overset(NaNO(2)+HCl)(to)Xoverset(N,N-"Dimean...
Text Solution
|
- Ethylamine is heated with CS(2) in the presence of Hg CI(2) The produc...
Text Solution
|
- Leakage of which gas was responsible for the Bhopal tragedy in 1984
Text Solution
|
- The aromatic compound would be :
Text Solution
|