A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise REVISION QUESTION FROM COMPETITIVE EXAMS|124 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQs|4 VideosPHYSICAL AND CHEMICAL EQUILIBRIA
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|13 VideosP BLOCK ELEMENTS (GROUP 13 AND 14 )
DINESH PUBLICATION|Exercise Straight obj.|17 VideosRATES OF REACTIONS AND CHEMICAL KINETICS
DINESH PUBLICATION|Exercise Ultimate Preparatory Package|29 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PHYSICAL AND CHEMICAL EQUILIBRIA-Multiple CHOICE QUESTIONS [Based on Numerical Problems]
- For the equilibrium 2H(2)O(g) hArr 2H(2)(g)+O(2)(g), equilibrium const...
Text Solution
|
- For the following equilibrium reaction N(2)O(4)(g)hArr 2NO(2)(g), NO(...
Text Solution
|
- There is 50% dimer formation of benzoic acid (C(6)H(5)COOH)(2) in benz...
Text Solution
|
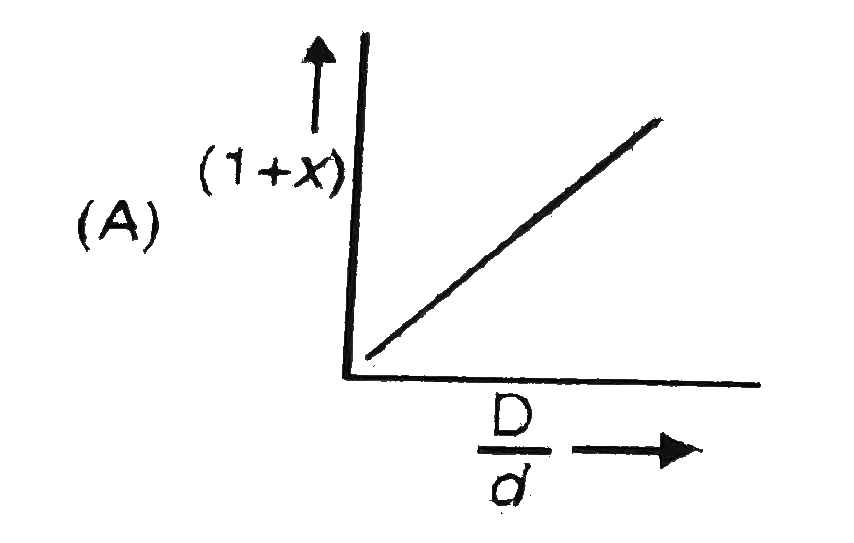
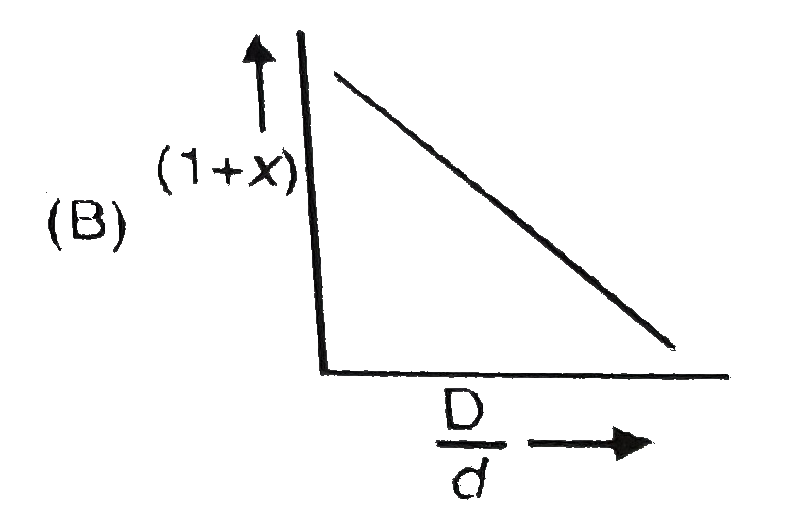
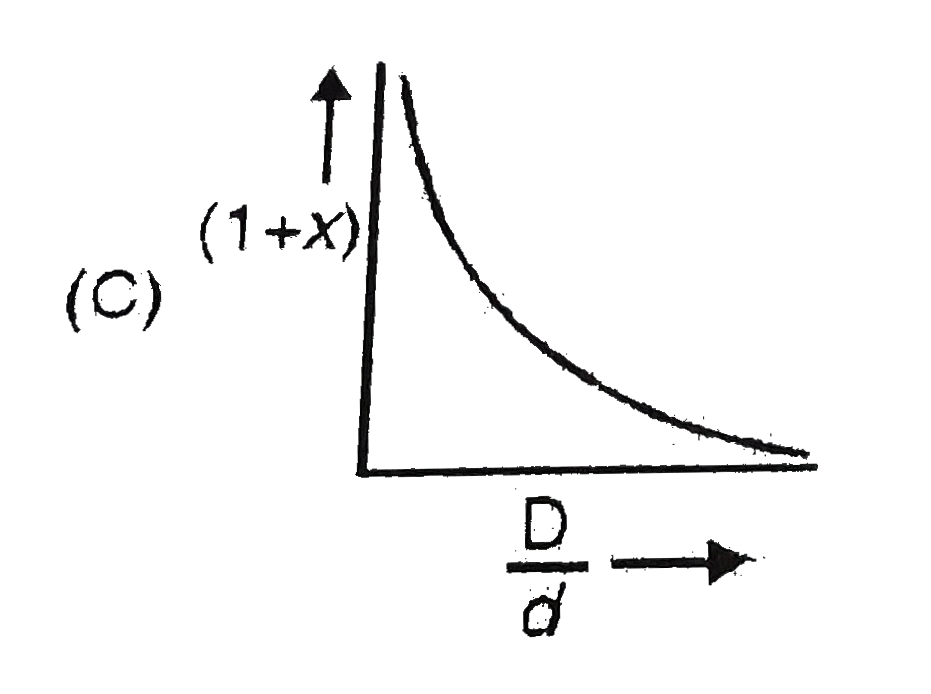
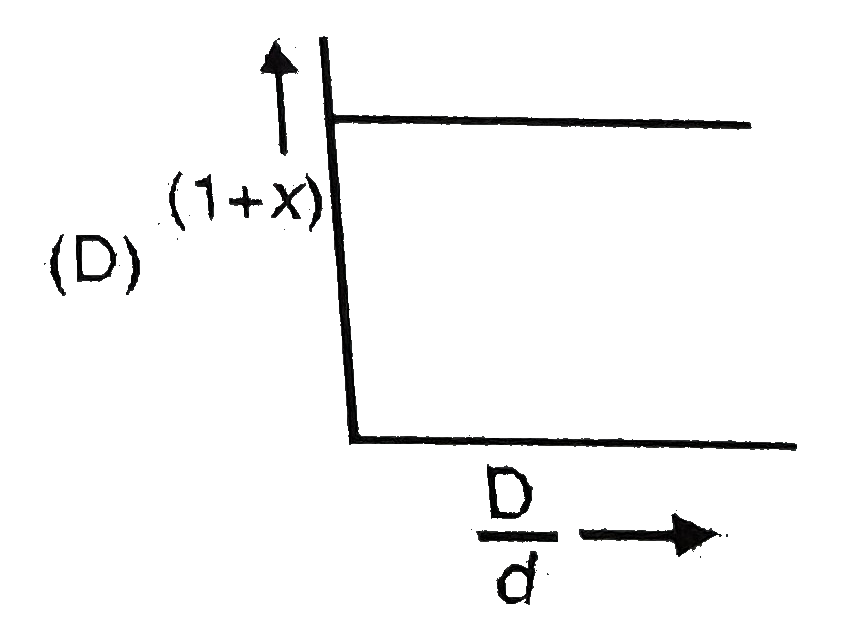
- In the dissociation of N(2)O(4) into NO(2). (1+x) values with the vapo...
Text Solution
|
- For the dissociation of PCl(5) into PCl(3) and Cl(2) in gaseous phase ...
Text Solution
|
- For a very small extent of dissociation of PCl(5) into PCl(3) and Cl(2...
Text Solution
|
- Before equilibrium is set-up for the chemical reaction, N(2)O(4)hArr 2...
Text Solution
|
- 1 mole each of CO(g), H(2)O(g), H(2)(g) and CO(2)(g) are placed in one...
Text Solution
|
- 60g CH(3)COOH and 46g C(2)H(5)OH react in 5L flask to form 44g CH(3)CO...
Text Solution
|
- One mole of N(20O(4)(g) at 300K is kept in a closed container under on...
Text Solution
|
- For the equilibrium 2SO(2)+O(2)hArr 2SO(3) we start with 2 moles of S...
Text Solution
|
- Sulphide ion (S^(2-)) reacts with solid sulphur forming S(2)^(2-) and ...
Text Solution
|
- The equilibrium constant for the reaction H(3)BO(3) + glycerin hArr (H...
Text Solution
|
- 1 mole of PCl(5) taken at 5 atm, dissociates into PCl(3) and Cl(2) to...
Text Solution
|
- For the following equilibrium N(2)O(4) hArr 2NO(2) K(c)=0.67. If we st...
Text Solution
|
- CaCO(3)(s) hArr CaO(s)+CO(2)(g) For the equilibrium , it is found th...
Text Solution
|
- For the following equilibrium : NH(2)CO(2)NH(4)(s)hArr 2NH(3)(g)+CO...
Text Solution
|
- In each of the following, total pressure set - up at equilibrium is as...
Text Solution
|
- For the equilibrium CuSO(4)xx5H(2)O(s)hArrCuSO(4)xx3H(2)O(s) + 2H(2)O(...
Text Solution
|
- At what relative humidity with Na(2)SO(4) be deliquescent (absorb mois...
Text Solution
|