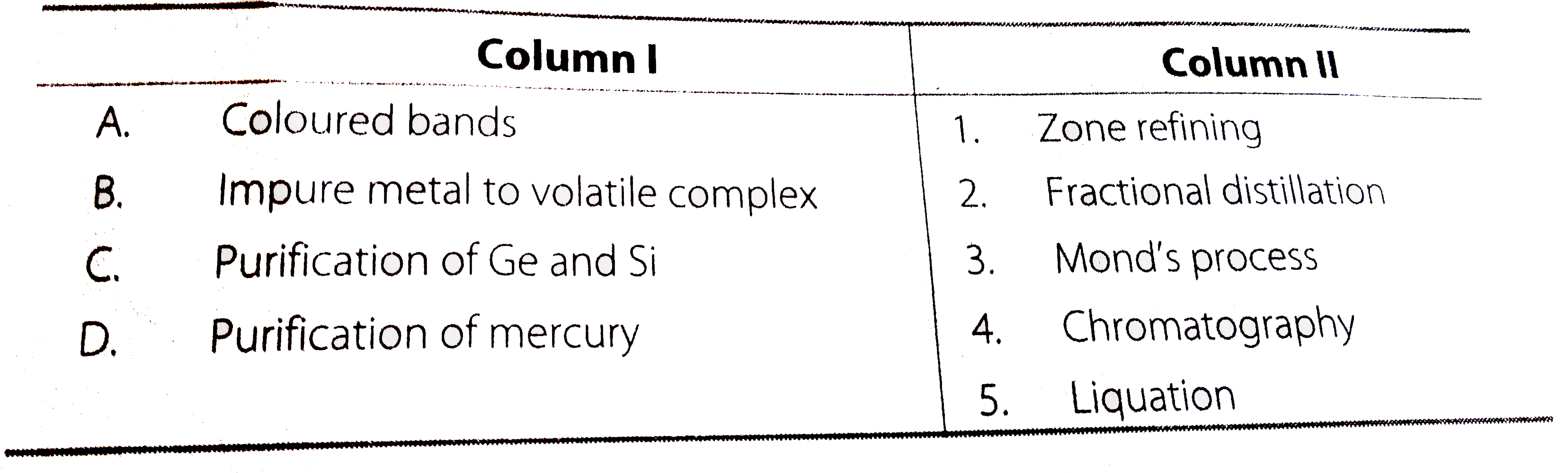
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-GENERAL PRINCIPLE AND PROCESSES OF ISOLATION OF ELEMENTS -General Principle And Processes Of Isolation Of Elements
- Why is sulphide ore of copper heated in a furnace afer mixing with sil...
Text Solution
|
- Why are sulphide ores converted to oxide before reduction?
Text Solution
|
- Which method is used for refining Zr and Ti? Explain with equation.
Text Solution
|
- What should be the considerations during the extraction of metals by e...
Text Solution
|
- What is the role of flux in metallurgical processes?
Text Solution
|
- How are metals used as semiconductor refined? What is the principle of...
Text Solution
|
- Write down the reactions taking place in Bast furnace related to the m...
Text Solution
|
- Give two requirements for vapour phase refining.
Text Solution
|
- Write the chemical reaction involved in the extraction of gold by cyan...
Text Solution
|
- Match the items of Column I with item of Column II and assign the corr...
Text Solution
|
- Match the items of Column I with item of Column II and assign the corr...
Text Solution
|
- Match the items of Column I with item of Column II and assign the corr...
Text Solution
|
- Match the items of Column I with item of Column II and assign the corr...
Text Solution
|
- Match the items of Column I with item of Column II and assign the corr...
Text Solution
|
- Assertion : Nickel can be purified by Mond process. Reason : Ni(CO)(...
Text Solution
|
- Assertion : Zirconium can be purificed by Van Arkel method. Reason :...
Text Solution
|
- Assertion : Sulphide ores are concentrated by Froth Floatation method....
Text Solution
|
- Assertion : Zone refining method is very useful for producing semicond...
Text Solution
|
- Assertion : Hydrometallurgy involves dissolving the ore in a suitable ...
Text Solution
|
- Explain the following (a) CO(2) is a better reducing agent below 7...
Text Solution
|