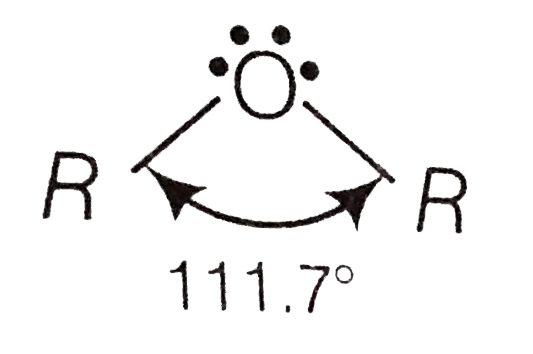
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ALCOHOLS, PHENOLS AND ETHERS-Alcohols, Phenols And Ethers
- The carbon-oxygen bond in phenol is slightly stronger than that in met...
Text Solution
|
- Arrange water, ethanol and phenol in increasing order of acidity and g...
Text Solution
|
- Match the structures of the compounds given in Column I with the name ...
Text Solution
|
- Match the starting material given in Column I with the products formed...
Text Solution
|
- Match the items of Column I with items of Column II.
Text Solution
|
- Match the items of Column I with items of Column II.
Text Solution
|
- Assertion (A) Addition reaction of water to but-1-ene in acidic medium...
Text Solution
|
- Assertion (A) p-nitrophenol is more acidic than phenol. Reason (R) N...
Text Solution
|
- Assertion (A) IUPAC name of the compound CH(3)-underset(CH(3))unders...
Text Solution
|
- Assertion (A) Bond angle is ethers is slightly less than tetrahedral a...
Text Solution
|
- Assertion (A) Boiling points of alcohols and ethers are high. Reason...
Text Solution
|
- Assertion (A) Like bromination of benzene, bromination of phenol is al...
Text Solution
|
- Assertion (A) o-nitrophenol is less soluble in water than the m and p-...
Text Solution
|
- Assertion (A) Ethanol is a weaker acid than phenol. Reason (R) Sodiu...
Text Solution
|
- Assertion (A) Phenol forms 2, 4, 6-tribromophenol o treatement with Br...
Text Solution
|
- Assertion (A) Phenols give o-and p-nitrophenol on nitration with conc....
Text Solution
|
- Write the mechanism of the reaction of HI with methoxybenzene.
Text Solution
|
- (a) Name the starting material used in the industrial preparation of p...
Text Solution
|
- How can phenol be converted to aspirin ?
Text Solution
|
- Explain a process in which a biocatalyst is used industrial preparatio...
Text Solution
|