Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Matching the columns|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Assertions and Reasons|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Long Answer type questions|9 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR|Exercise Long answer types question|7 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Short answer types questions
- Using molelcular orbital theory, compare the bond energy and magnetic ...
Text Solution
|
- Explain the shape of BrF(5^.)
Text Solution
|
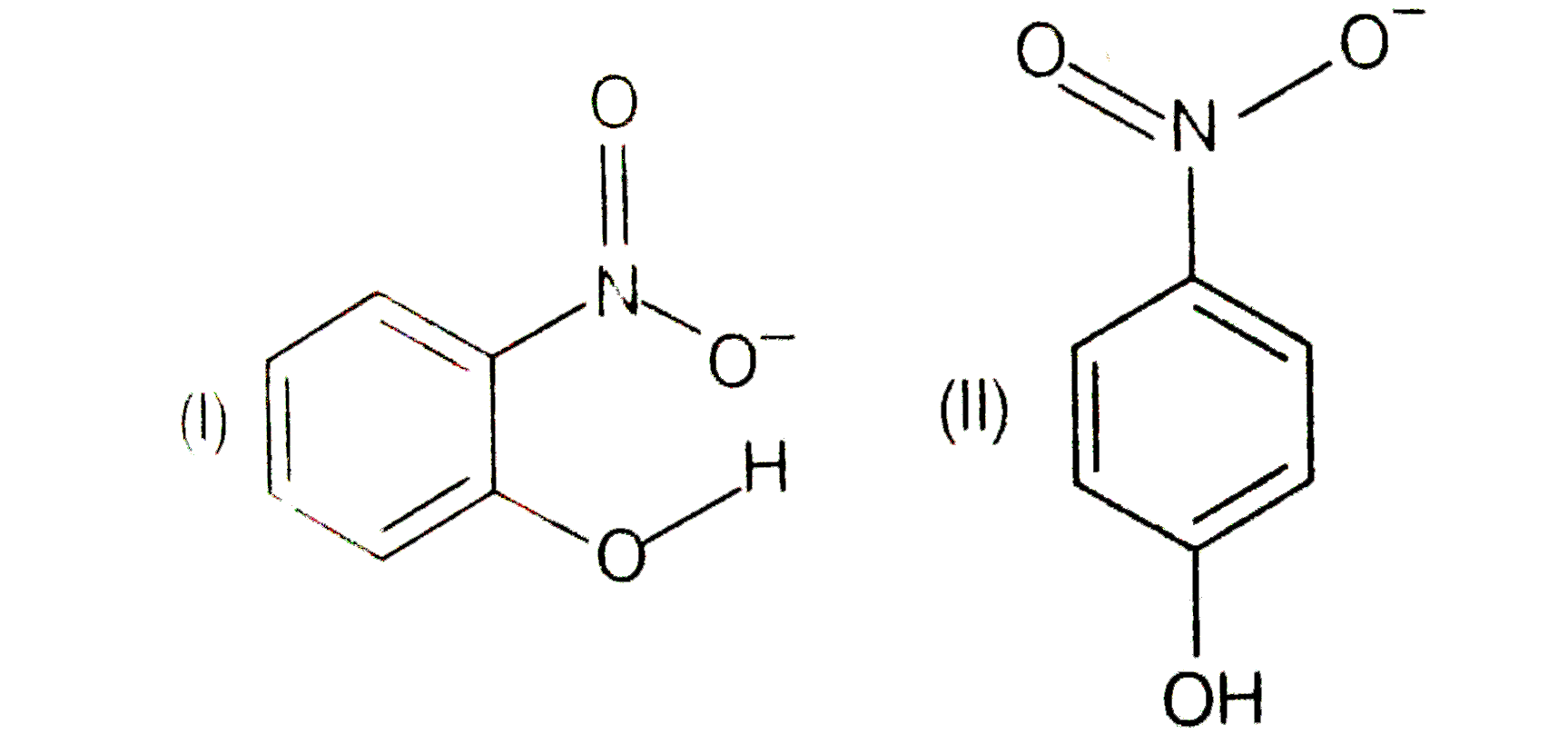
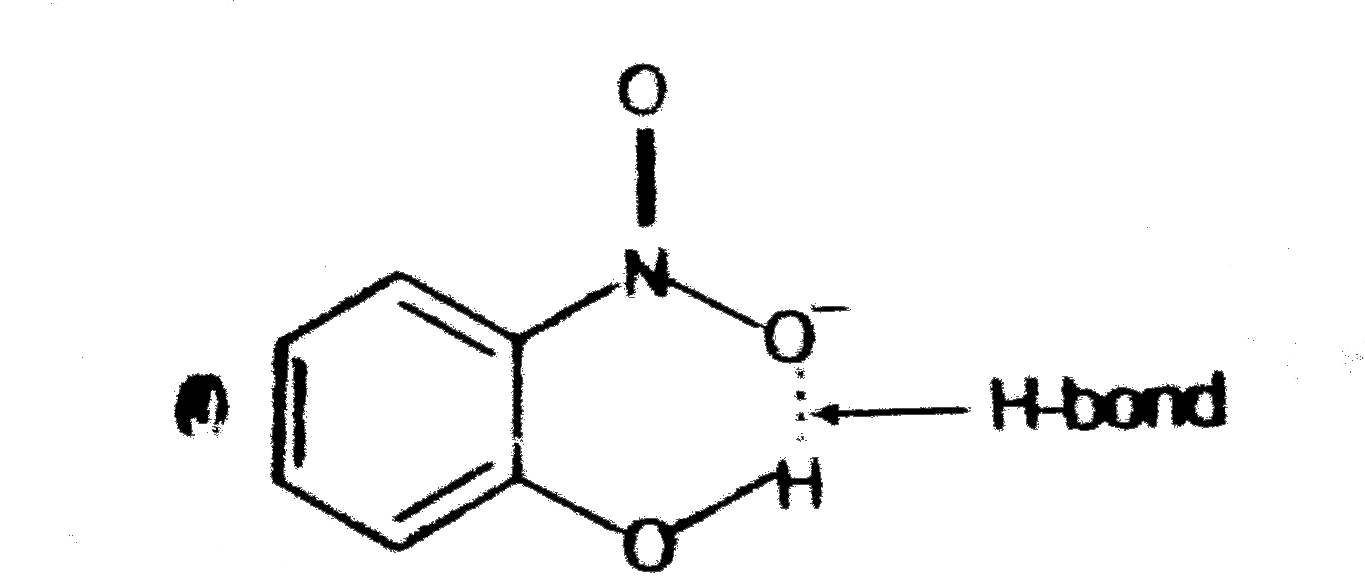
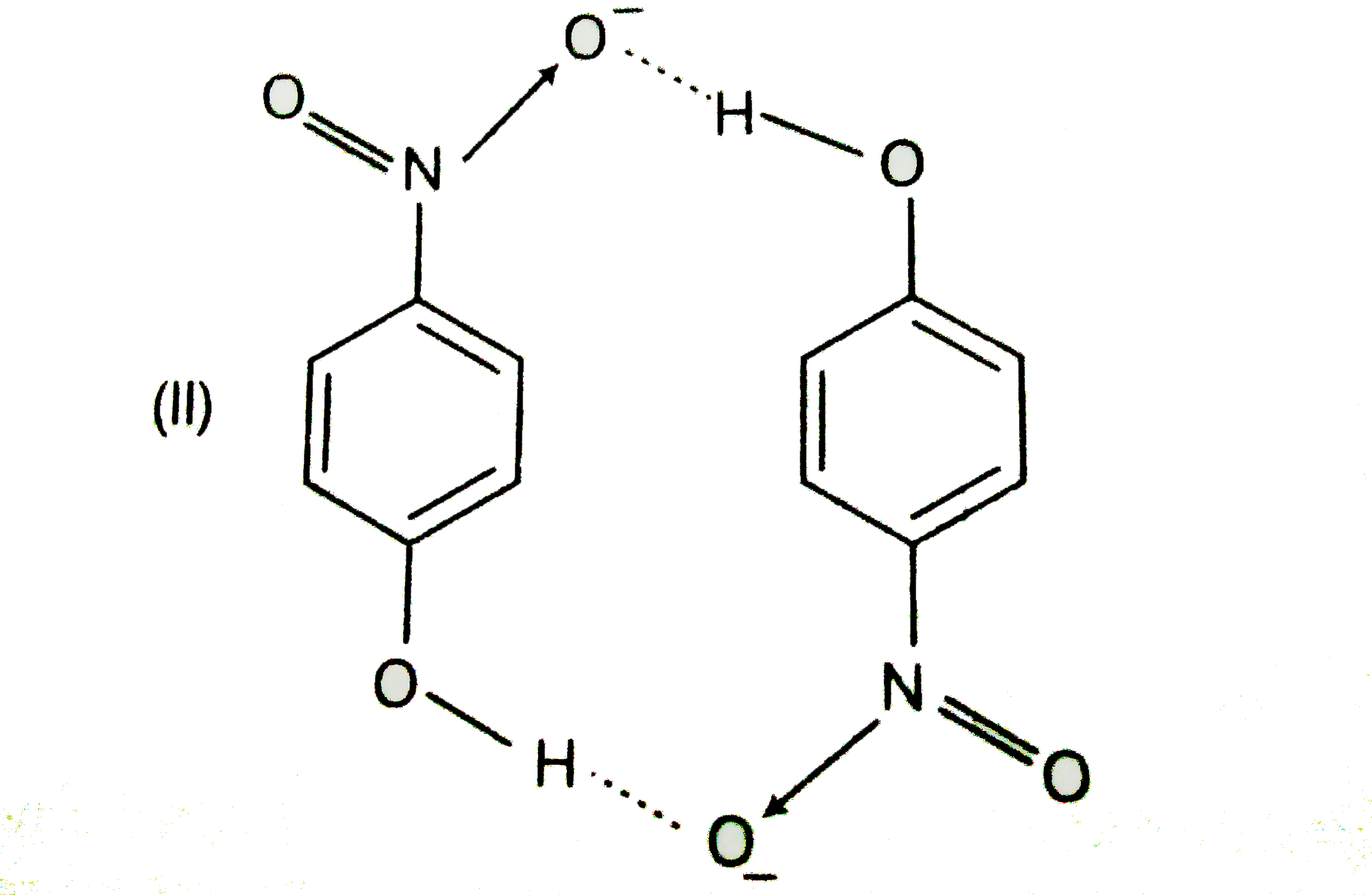
- Structures of molecules of two compounds are given below. a) Whic...
Text Solution
|
- Why does type of overlap given in the following figure not result in b...
Text Solution
|
- Explain why PCl(5) is trigonal bipyramidal whereas IF(5) is square pyr...
Text Solution
|
- In both water and dimethyl ether (CH(3)-underset(..)overset(..)O-CH(3)...
Text Solution
|
- Write Lewis structure of the following compounds and show format charg...
Text Solution
|
- The energy of sigma2p(z), molecular orbital is greater than pi2p(x) an...
Text Solution
|
- Give the change in bond order in the following ionisation process? i...
Text Solution
|
- Give reason for the following. a) Covalent bonds are directional bon...
Text Solution
|
- What is an ionic bond? With two suitable exmaples the difference betwe...
Text Solution
|
- Arrange the following bonds in order of increasing ionic character giv...
Text Solution
|
- Explain why CO(3)^(2-) ion cannot be represented by a single Lewis str...
Text Solution
|
- Predict the hybridisation of each carbon in the molecule of organic co...
Text Solution
|
- Group the following as linear and non-linear molecules : H(2)O,HOCl,...
Text Solution
|
- Elements X,Y and Z have 4,5 and 7 valence electrons respectively, (i) ...
Text Solution
|
- Draw the resonatin structure of (i) Ozone molecule (ii) Nitrate ion
Text Solution
|
- Presict the shapes of the following molecules on the basis of hybridis...
Text Solution
|
- All the C-O bonds in carbonate in (CO(3)^(2-)) are equal in length. Ex...
Text Solution
|
- what is meant by the term average bond enthalpy? Why is there differen...
Text Solution
|