Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
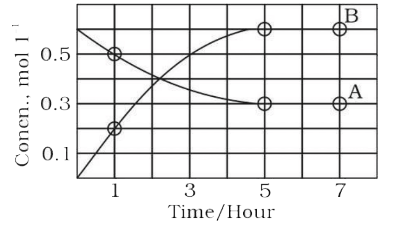
- The progress of the reaction, A ⇌ nB with time, is presented in figure...
Text Solution
|
- The progress of the reaction A hArr nB with time is persented in the f...
Text Solution
|
- The progress of the reaction A hArr nB , with times is presented in th...
Text Solution
|
- The process of the reaction A hArr nB with time is represented in the ...
Text Solution
|
- For a chemical reaction, variation in concentration [A] vs time (s) pl...
Text Solution
|
- The progress of the reaction AhArrnB with time, is presented in figure...
Text Solution
|
- The progress of the reaction AhArrnB with time, is presented in figure...
Text Solution
|
- The progress of the reaction AhArrnB with time, is presented in figure...
Text Solution
|
- The values of rate constants of some reactions are given below . Deter...
Text Solution
|