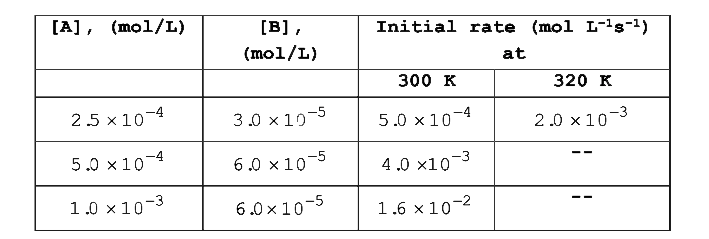Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data for the reaction between A and B Calculat...
Text Solution
|
- The rate constant of a reaction will be equal to the pre-exponential f...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- For a first order reaction A rarr P , the temperature (T) dependent ra...
Text Solution
|
- form the following data for the reaction between A and B, (a) Cal...
Text Solution
|
- From the following data for the reaction between A and B Calculat...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- For a first order reaction A rarr P, the temperature (T) dependent rat...
Text Solution
|
- From the following data for the reaction between A and B Calculate: (i...
Text Solution
|