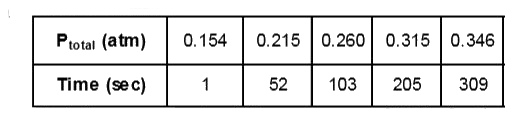
Text Solution
Verified by Experts
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise -1 (Introduction, Rate of reaction, Factor affecting rate of reaction, Effect of concentration on reaction rate)|9 VideosChemical Kinetics
MOTION|Exercise Exercise -1 (Zero order reaction, 1st order reaction , 2nd order reaction & nth order reaction)|11 VideosChemical Kinetics
MOTION|Exercise SOLVED EXAMPLE (OBJECTIVE)|10 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-SOLVED EXAMPLE (SUBJECTIVE)
- The reaction given below, rate constant for disappearance of A is 7.48...
Text Solution
|
- The net rate of reaction of the change : [Cu(NH(3))(4)]^(2+)+H(2)O ?...
Text Solution
|
- The net rate of reaction of the change : [Cu(NH(3))(4)]^(2+)+H(2)O ?...
Text Solution
|
- The net rate of reaction of the change : [Cu(NH(3))(4)]^(2+)+H(2)O ?...
Text Solution
|
- The rate law for the decompoistion of gaseous N(2)O(5), N(2)O(5)(g) ...
Text Solution
|
- The half time of first order decomposition of nitramide is 2.1 hour at...
Text Solution
|
- The half time of first order decomposition of nitramide is 2.1 hour at...
Text Solution
|
- The reaction A+OH^(-)rarr Products, obeys rate law expression as : (...
Text Solution
|
- A certain reaction A + B rarr Products is first order w.r.t. each reac...
Text Solution
|
- Dimethyl ether decomposes according to the following reaction : CH(3...
Text Solution
|
- The decomposition of N(2)O(5) according to following reaction is first...
Text Solution
|
- The gas phase decomposition of N(2)O(5)" to "NO(2) and O(2) is monitor...
Text Solution
|
- 5 ml of ethylacetate was added to a flask containing 100 ml f 0.1 N HC...
Text Solution
|
- The optical rotations of sucrose in 0.5N HCl" at "35^(@)C at various t...
Text Solution
|
- The hydrolysis of ethyl acetate CH(3)COOC(2)H(3)+H(2)O hArr CH(3) CO...
Text Solution
|
- Two first order reactions proceed at 25^(@)C at the same rate. The tem...
Text Solution
|
- For the reaction : C(2)H(5)I+OH^(-) rarr C(2)H(5)OH + I^(-) the ra...
Text Solution
|
- A secondary alkyl halide (RX) is hydrolysed by alkali simultaneously b...
Text Solution
|
- A polymerisation reaction is carried out at 2000 K and the same reacti...
Text Solution
|
- Consider the following first order parallel reaction. The concent...
Text Solution
|