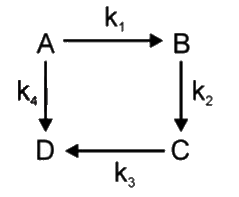
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise - 2 (Level-I) (Introduction, Rate of reaction, Factor affecting rate of reaction, Effect of)|14 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-I) (Zero order reaction, 1st order reaction, 2nd order reaction & nth order reaction)|12 VideosChemical Kinetics
MOTION|Exercise Exercise -1 (Mechanism of reaction and theories of reaction rate)|6 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise -1 (Sequential, parallel & reversible reaction)
- For a reaction,net rate is ((dx)/(dt))=k[A]^(2)-k'[C][B]^(2) then ,Sel...
Text Solution
|
- Consider the elementary reaction sequence shown in figure. Which of th...
Text Solution
|
- For a reaction of reversible nature , net rate is ((dx)/(dt))=k(1)[A]^...
Text Solution
|
- At a given temperature ,k(1)=k(2) for the reaction, A+BhArrC+D If [...
Text Solution
|
- The substance undergoes first order decomposition. The decomposition f...
Text Solution
|
- The rate constant for two parallel reactions were found reactions were...
Text Solution
|
- An organic compound dissociates into n parallel first order reactions ...
Text Solution
|