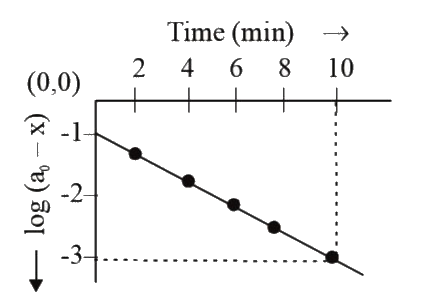
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 2)|26 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 3)|4 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 1)|3 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise - 2 (Level-II) (Zero order reaction, 1st order reaction, 2nd order reaction & nth order reaction)
- In an elementary reaction A(g)+2B(g) to C(g) the initial pressure of A...
Text Solution
|
- Which graph represents zero-order reaction [A(g) rarr B (g)] ?
Text Solution
|
- The rate of decomposition of NH(3)(g) at 10 atm on platinum surface is...
Text Solution
|
- A overset(k) rarrB, t(1//2)=10" min "3A rarrC Both reaction have sam...
Text Solution
|
- The gas phase decomposition (in closed container) 2A((g)) rarr 4B((g...
Text Solution
|
- If decompositon reaction A(g)toB(g) follows first order linetics then ...
Text Solution
|
- For the first order decomposition of SO(2)Cl(2)(g), SO(2)Cl(2)(g) rarr...
Text Solution
|
- SO(3) gas is entering the environment at a constant rate of 6.93 xx 10...
Text Solution
|
- Which of the following statements are correct about half life period ?
Text Solution
|