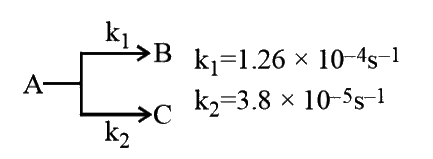A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 5)|1 VideosChemical Kinetics
MOTION|Exercise Exercise - 3|72 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 3)|4 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise - 2 (Level-II) (COMPREHENSION - 4)
- The rate of reaction ((dx)/(dt)) varies with nature, physical state an...
Text Solution
|
- The rate of a reaction ((dx)/(dt)) varies with nature, physical state ...
Text Solution
|
- The rate of reaction ((dx)/(dt)) varies with nature, physical state an...
Text Solution
|
- Consider the following energy diagram for the reaction. A(2)+B(2) ?...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|