Text Solution
Verified by Experts
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise - 4 (Level - I)|18 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II)|15 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-II) (COMPREHENSION - 5)|1 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise - 3
- A vessel contains dimethyl ether at a pressure of 0.4 atm. Dimethyl et...
Text Solution
|
- For the two parallel reactions A overset(k(1)) rarr B and A overset(k(...
Text Solution
|
- The energy of activation of a first order reaction is 104.5" kJ mole"^...
Text Solution
|
- The specific rate constant for a reaction increases by a factor of 4, ...
Text Solution
|
- The energy of activation and specific rate constant for a first order ...
Text Solution
|
- A 1st order reaction is 50% complete in 30 minute at 27^(@)C and in 10...
Text Solution
|
- A catalyst lowers the activation energy for a certain reaction from 75...
Text Solution
|
- Given that the temperature coefficient for the saponification of ethyl...
Text Solution
|
- The rate constants of a reaction at 500 K and 700 K are 0.02 s^(–1) an...
Text Solution
|
- For a reaction ,calculate value of ratio, ([x](t))/([y]+[z]) at any g...
Text Solution
|
- k(1)=xhr^(-1), k(1): k(2) =1:10. Calculate ([C])/([A]) after one hour ...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- For a reaction A rarr B rarr C (t1//2) for A & B are 4 and 2 minutes r...
Text Solution
|
- For the following first order gaseous reaction The initial pressu...
Text Solution
|
- The reaction, 2NO+Br(2)rarr 2NOBr, is supposed to follow the following...
Text Solution
|
- For the reaction 2H(2) + 2NO rarr N(2) + 2H(2)O, the following mechani...
Text Solution
|
- Reaction between NO and O(2)" to form "NO(2)" is "2NO + O(2) rarr 2NO...
Text Solution
|
- For the mechanism A+B ? K(2) C, C overset(k(3)) rarr D (a) Derive th...
Text Solution
|
- The reaction of formation of phosgene from CO and Cl(2)" is "CO + Cl(2...
Text Solution
|
- The approach to the following equilibrium was observed kinetically fro...
Text Solution
|
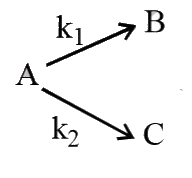 , `k_(1)=1.26xx10^(-4)" sec"^(-1) and k_(2)=3.6xx10^(-5)" sec"^(-1)` Calculate the % distribution of B & C.
, `k_(1)=1.26xx10^(-4)" sec"^(-1) and k_(2)=3.6xx10^(-5)" sec"^(-1)` Calculate the % distribution of B & C.