A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LIQUID SOLUTION
MOTION|Exercise EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED|30 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-3 SUBJECTIVE JEE ADVANCED|40 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-1 OBJECTIVE PROBLEMS JEE MAIN|68 VideosISOMERISM
MOTION|Exercise Exercise 4|26 VideosMETALLURGY
MOTION|Exercise EXERCISE 4 (LEVEL - II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-LIQUID SOLUTION-EXERCISE-2 (LEVEL -I) OBJECTIVE PROBLEMS JEE MAIN
- The solubility of gases in liquids:
Text Solution
|
- The solubility of N(2)(g)in water exposed to the atmosphere, when the ...
Text Solution
|
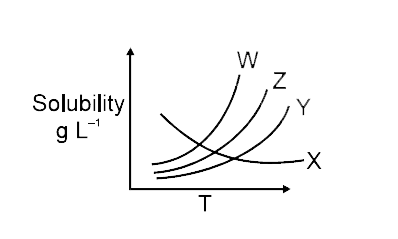
- Solubility curves of four ionic salts X, Y, Z, W are given below : ...
Text Solution
|
- Select in correct statements :
Text Solution
|
- Select incorrect statement :
Text Solution
|
- The vapour pressure of water depends upon :
Text Solution
|
- Among the following substances, the lowest vapour pressure is exerted ...
Text Solution
|
- A sample of air saturated with benzene (vapour pressure = 100 mm Hg at...
Text Solution
|
- At a given temperature total vapour pressure (in Torr) of a mixture of...
Text Solution
|
- For an ideal binary liquid solutions with P(A)^(@)gtP(B)^(@), which re...
Text Solution
|
- Molal fraction of A vapours above te solution in mixture of A and B ["...
Text Solution
|
- Two liquid A & B form an ideal solution. What is the vapour pressure o...
Text Solution
|
- Total vapour pressure of mixture of 1 mol of volatile component A(P(A^...
Text Solution
|
- Which of the following is less than zero for ideal solutions ?
Text Solution
|
- Identify the false statement :
Text Solution
|
- Consider a binary mixture of volatile liquides. If at X(A)=0.4, the va...
Text Solution
|
- The diagram given below represents boiling point composition diagram o...
Text Solution
|
- The van't Hoff factor for 0.1 M Ba(NO(3))(2) solution is 2.74. The deg...
Text Solution
|
- The Van't Hoff factor for a dillute aqueous solution of glucose is
Text Solution
|
- A complex containing K^(+),Pt(IV) and Cl^(--) is 100% ionised giving i...
Text Solution
|