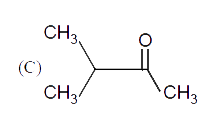
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED|21 VideosALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -3 OBJECTIVE PROBLEMS JEE ADVANCED|17 VideosALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -1 OBJECTIVE PROBLEMS JEE MAIN|47 VideosALKYI HALIDE
MOTION|Exercise Exercise - 4 | Level-II|8 Videos
Similar Questions
Explore conceptually related problems
MOTION-ALCOHOLS & ETHERS-EXERCISE -2 (LEVEL -I) OBJECTIVE PROBLEMS JEE MAIN
- A water soluble C6H(14)O2 ompound is oxidized by lead tetraacetate (o...
Text Solution
|
- A racemic mixture of (+-) 2-pheynl propanoic acid on esterification wi...
Text Solution
|
- Which of the following alcohol can not be oxidized by KMnO4
Text Solution
|
- Which of the following is wrong -
Text Solution
|
- Which of the following reacts fastest with conc. HCl ?
Text Solution
|
- Compare the rate of acid catalyst dehydration
Text Solution
|
- Major product 'P' is :
Text Solution
|
- CH3-overset(CH3)overset(|)underset(CH3)underset(|)C-CH2CH2CH2OH overse...
Text Solution
|
- Identify the correct statement about reaction and products
Text Solution
|
- overset(H^(oplus))underset(Delta)to Major product Major product is
Text Solution
|
- Ph-overset(Ph)overset(|)underset(H)underset(|)C-overset(H)overset(|)un...
Text Solution
|
- Compare the rate of dehydration of the following alcohols (I) Ph-under...
Text Solution
|
- CH3-overset(CH3)overset(|)underset(OH)underset(|)C-overset(CH3)overset...
Text Solution
|
- Incorrect statement is
Text Solution
|
- Find out the product :
Text Solution
|
- Which of the following is the product from ethanol addition to dihydro...
Text Solution
|
- What product (s) are expected from the following reaction ?
Text Solution
|
Text Solution
|
Text Solution
|
- In the given reaction P will be
Text Solution
|