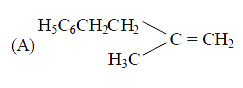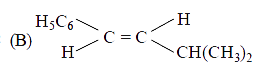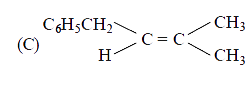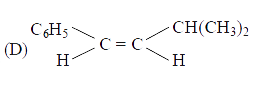A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ALCOHOLS & ETHERS-EXERCISE -4 (LEVEL -I) PREVIOUS YEAR JEE MAIN
- HBr reacts with H(2)C=CH-OCH(3) under anhydrous conditions at room tem...
Text Solution
|
- CH(3)CH(2)OH overset(P+I(2))to Aunderset("ether")overset("Mg")to B ove...
Text Solution
|
- A liquid was mixed with ethanol and a drop of concentrated H(2) SO(4) ...
Text Solution
|
- From amongest the following alcohols, the one that would react fastest...
Text Solution
|
- The main product of the following reaction is C6H5CH2CH(OH)CH(CH3)2 ov...
Text Solution
|
- Consider the following reaction, C(2)H(5)OH+H(2)SO(4)rarr Product ...
Text Solution
|
- The most suitable reagent for the conversion of R- CH(2) - OH rarr R...
Text Solution
|
- 2-chloro-2-methylpentane on reaction with sodium methoxide in methanol...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The increasing order of the boiling points for the following compounds...
Text Solution
|
- In the following reaction sequence The compound I is
Text Solution
|
- Phenol reacts with methyl chloroformate in the presence of NaOH to for...
Text Solution
|