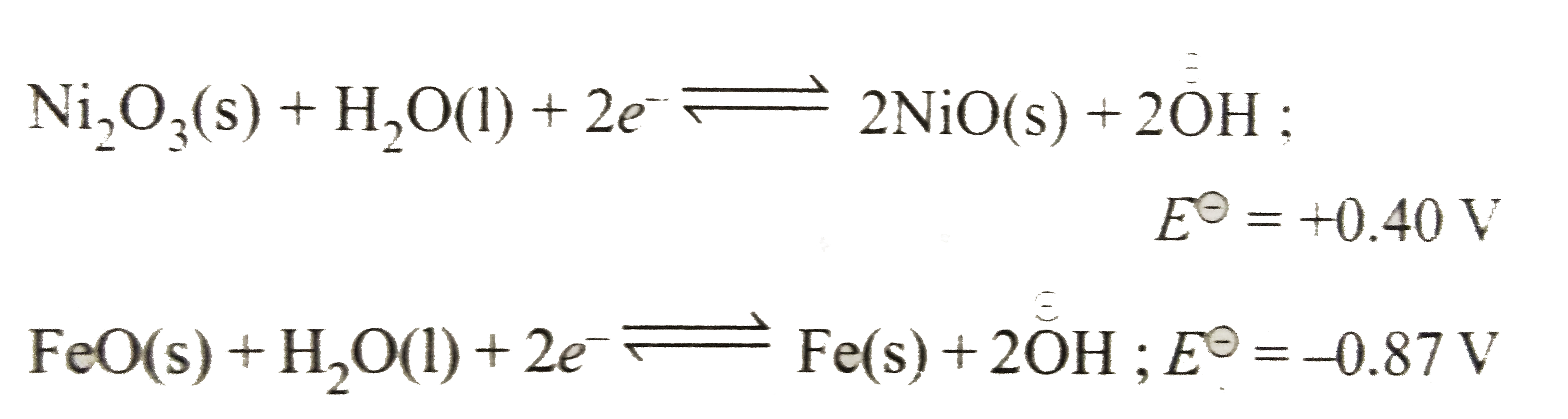Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ELECTROCHEMISTRY-EXERCISE-3
- Calculate the e.m.f. of the cell Pt|H(2)(1.0atm)|CH(3)COOH (0.1M)||N...
Text Solution
|
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- The Edison storage cell is represented as Fe(s)|FeO(s)|KOH(aq)|Ni2O3...
Text Solution
|
- The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t...
Text Solution
|
- Determine the degree of hydrolysis and hydrolysis constant of aniline ...
Text Solution
|
- The emf of the cell, Pt|H2(1 atm),|H^+(0.1M,30ml)||Ag^+(0.8M)|Ag is 0....
Text Solution
|
- The emf of the cell Ag|AgI|KI(0.05M)||AgNO3(0.05M)|Ag is 0.788 V. Ca...
Text Solution
|
- Consider the cell AG|AgBr(s)|Br^(-)||AgCI(s)|CI^(-)|Ag at 25^(@)C. The...
Text Solution
|
- The pK(sp) of AgI is 16.07 if the E^(@) value for Ag^(+)//Ag is 0.7991...
Text Solution
|
- For the galvanic cell : Ag|AgCl(s)),KCl(0.2M)||KBr(0.001M),AgBr(s)|A...
Text Solution
|
- Given E^0=-0.268V for the Cl^-|PbCl2|Pb couple and -0.126 V for the Pb...
Text Solution
|
- Calculate the voltage E of the cell at 25^(@)C Mn(s) |Mn(OH(2))(s)|M...
Text Solution
|
- Calculate the voltage,E, of the cell Ag(s)|AgIO3(s)|Ag^+(x M),HIO3(0...
Text Solution
|
- Estimate the cell potential of a Daniel cell having 1.0Zn^(++) and ori...
Text Solution
|
- The overall formation constant for the reacction of 6 mol of CN^- with...
Text Solution
|
- Calculate E^0 for the following reactions at 298K, Ag(NH3)2^+ +e^- ...
Text Solution
|
- Calculate the equilibrium constant for the reaction: 3Sn(s) +2Cr(2)O...
Text Solution
|
- Calculate the equilibrium concentration of all ions in an ideal soluti...
Text Solution
|
- One of the methods of preparation of per disulphuric acid, H(2)S(2)O(8...
Text Solution
|