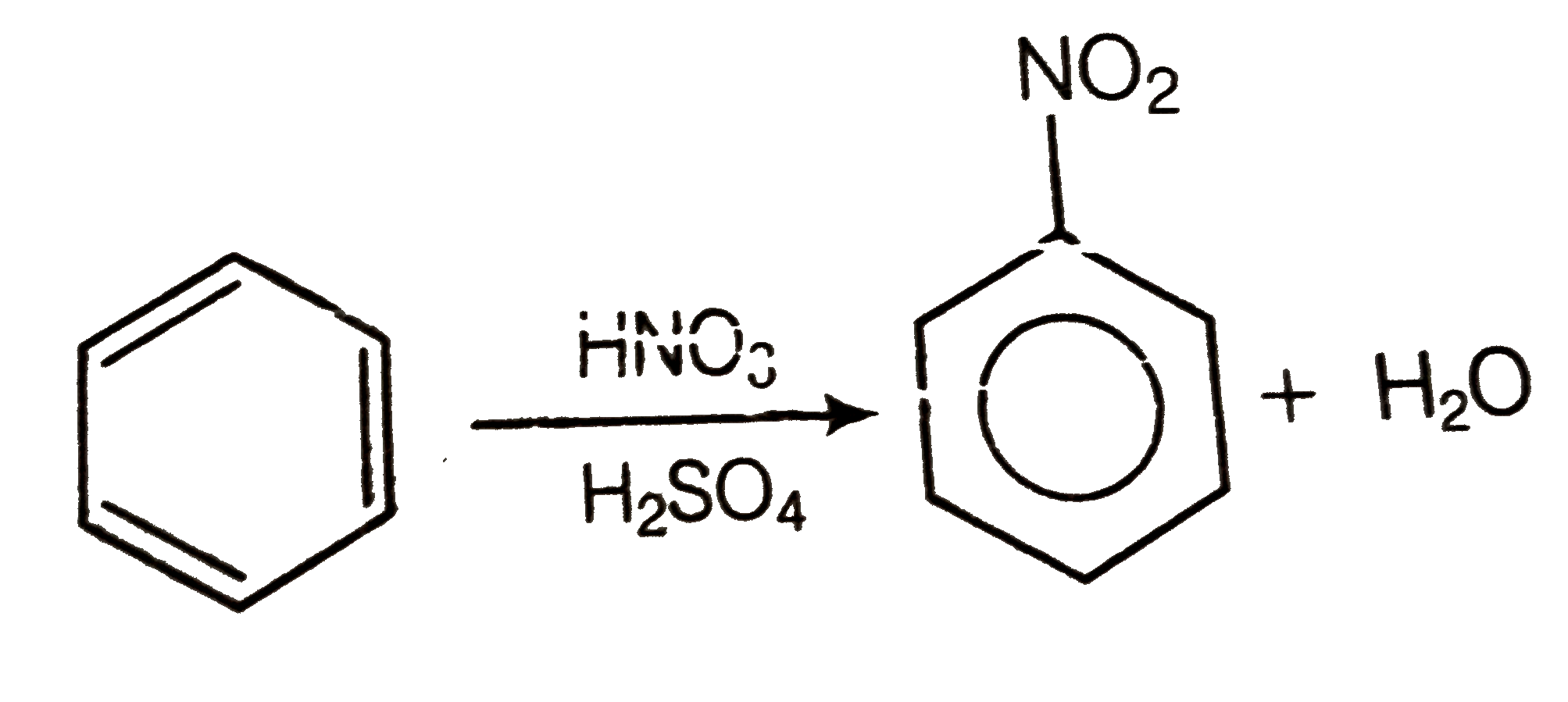
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-HYDROCARBONS-Assertion and Reason
- Assertion (A) The compound tetraene has the following structural formu...
Text Solution
|
- Assertion (A) Toluene on Friedal Crafts methylation gives o - and p-xy...
Text Solution
|
- S-I: Nitration of benzene with nitric acid requires the use of concent...
Text Solution
|
- Assertion (A) Amon isomeric isomeric pentanes, 2, 2-dimethylpentane ha...
Text Solution
|