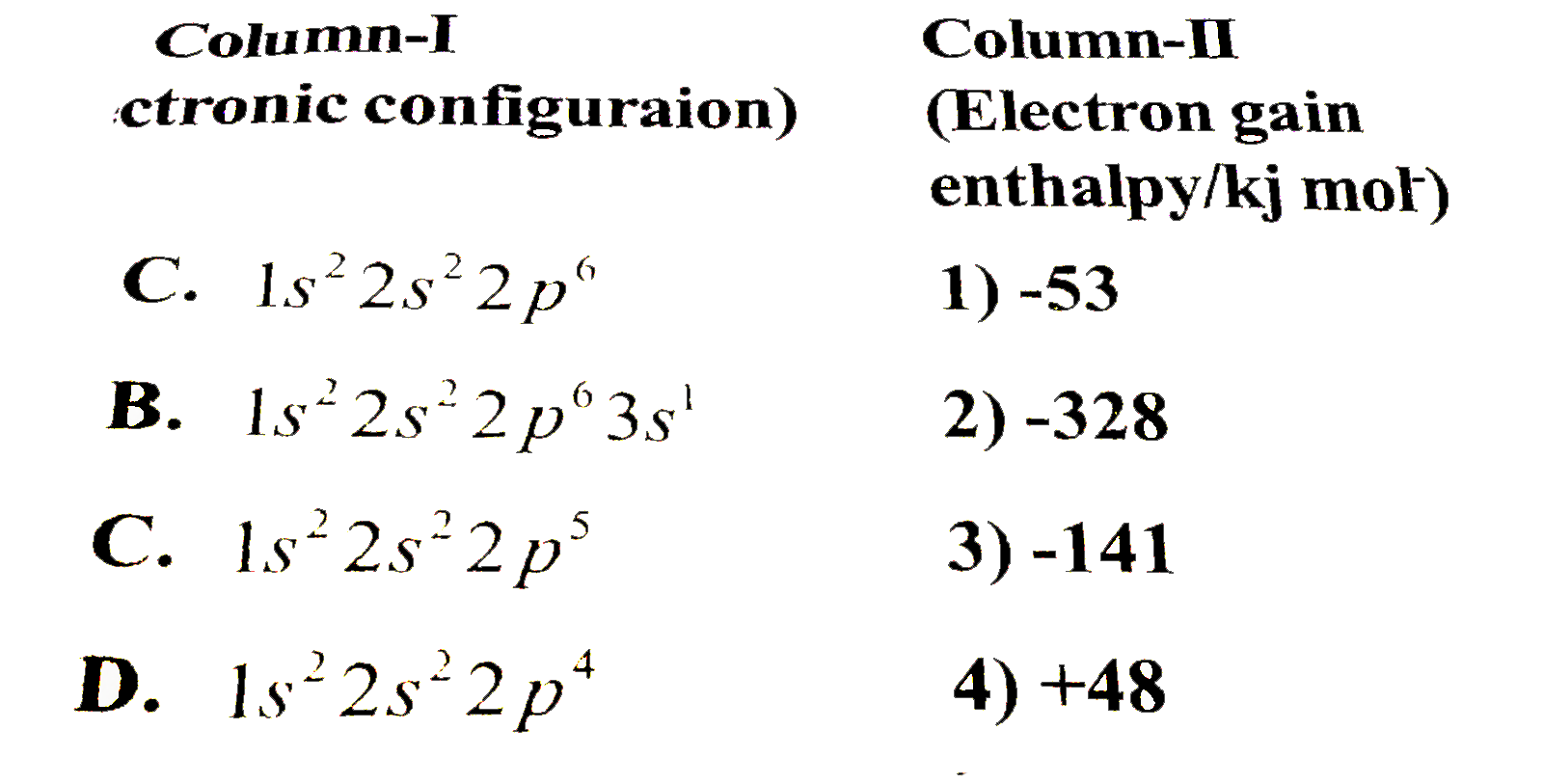Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR|Exercise Assertion and Reason|3 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR|Exercise Long answer types question|7 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR|Exercise Short answer types questions|19 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Long Answer type questions|9 VideosENVIRONMENTAL CHEMISTRY
NCERT EXEMPLAR|Exercise Long Answer Type|5 Videos
Similar Questions
Explore conceptually related problems