A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-SOLUTIONS -Solutions
- Value of Henry's constant K(H)…
Text Solution
|
- The value of Henry's constant K(H).
Text Solution
|
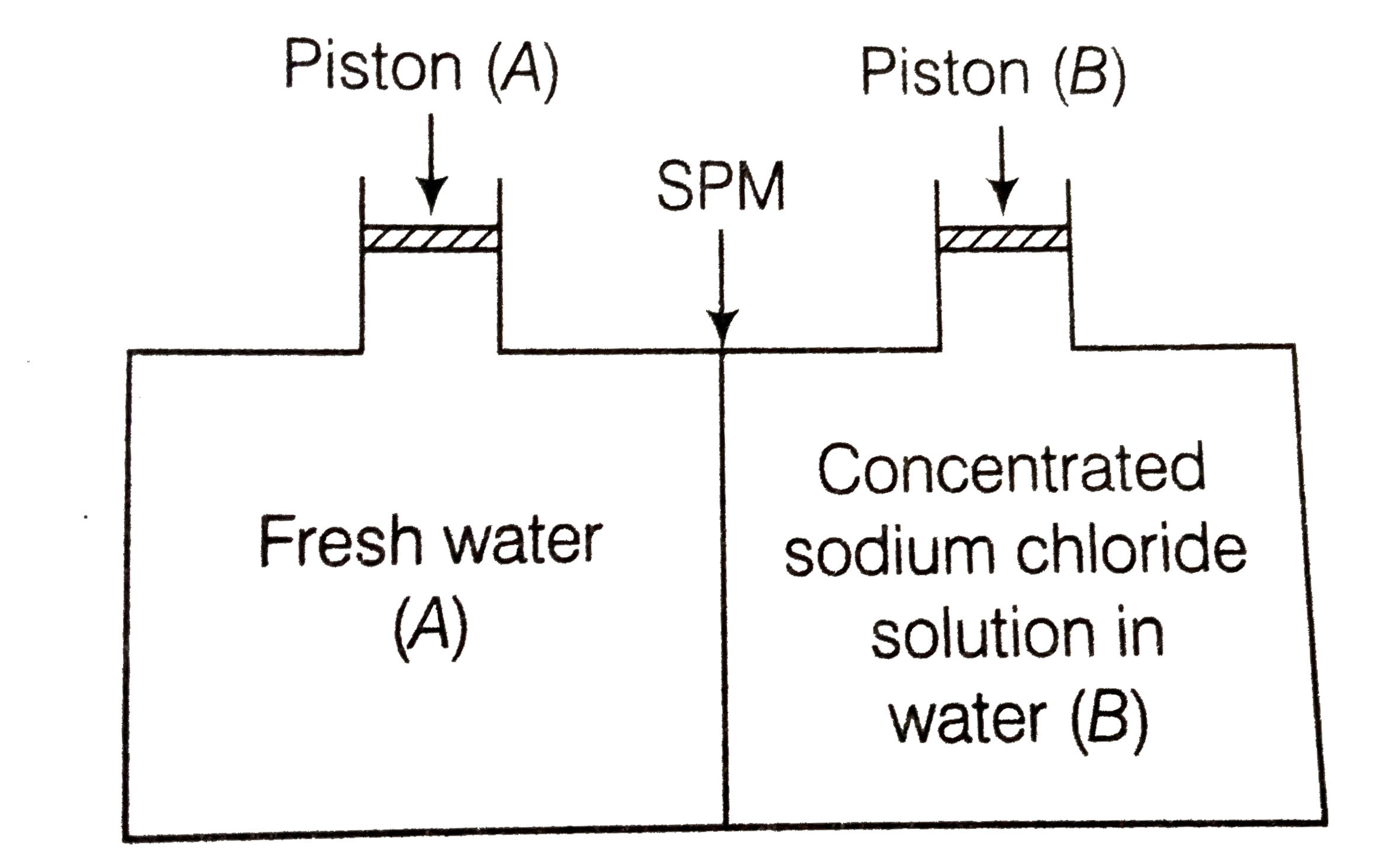
- Consider the figure and mark the correct option.
Text Solution
|
- We have three aqueous solutions of NaCl labelled as A, B and C with co...
Text Solution
|
- On the basic of information given below mark the Correct option .Infor...
Text Solution
|
- Two beakers of capacity 500 mL were taken. One of these beakers, label...
Text Solution
|
- If two liquids A and B from minimum boiling azeotrope at some specific...
Text Solution
|
- 4 L of 0.02 M aqueous solution of NaCl was diluted by adding 1 L of wa...
Text Solution
|
- On the basis of information given below mark the correct option. Inf...
Text Solution
|
- K(H) value for Ar(g),CO(2)(g),HCHO(g)and CH(4)(g) are 40.39,1.67,1.83x...
Text Solution
|
- Which of the following factor (s) affect the solubility of a gaseous s...
Text Solution
|
- Intermolecular forces between two benzene molecules are nearly of same...
Text Solution
|
- Relative lowering of vapour pressure is a colligative property because
Text Solution
|
- van't Hoff factor (i) is given by the expression
Text Solution
|
- Isotonic solutions must have the same………..
Text Solution
|
- Which of the following binary mixture will have same composition in li...
Text Solution
|
- In istonic solutions………..
Text Solution
|
- For a binary ideal liquid solution, the variation total vapour pressur...
Text Solution
|
- Colligative properties are observed when……..
Text Solution
|
- Components of a binarey mixture of two liquids A and B were being sepa...
Text Solution
|