A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-P-BLOCK ELEMENTS-P-Block Elements
- A black compound of manganese reacts with a halogen acid to give green...
Text Solution
|
- In the preparation of compounds of Xe, Bartlett has taken O(2)^(+)Pt F...
Text Solution
|
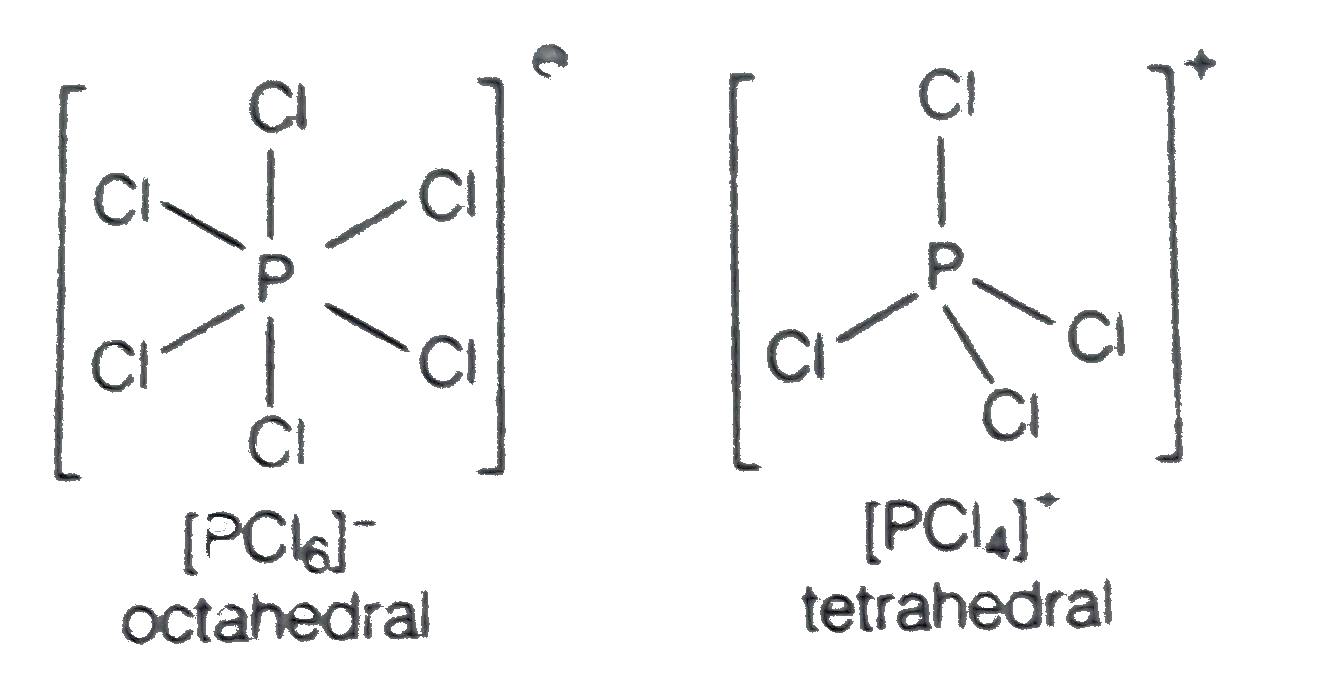
- In solid state, PCl(5) is a……..
Text Solution
|
- Reduction potentials of some ions are given below. Arrange them in dec...
Text Solution
|
- Which of the following is isoelectronic pair ?
Text Solution
|
- If chlorine gas is passed through hot NaOH solution, two changes are o...
Text Solution
|
- Which of the following options are not accordance with the property me...
Text Solution
|
- Which of the following is correct for P(4) molecule of white phosphoru...
Text Solution
|
- Which of the following statements are correct? I. Among halogens, ra...
Text Solution
|
- Which of the following statements are correct for SO(2) gas? (a) I...
Text Solution
|
- Which of the following statements are correct ? (a) All the three...
Text Solution
|
- Which of the following orders are correct as per the properties mentio...
Text Solution
|
- Which of the following statements are correct? (a) SS bond is prese...
Text Solution
|
- In which of the following reactions conc. H(2)SO(4) is used as an ox...
Text Solution
|
- Which of the following statements are true? (a) Only type of intera...
Text Solution
|
- In the preparation of H(2)SO(4) by Contact process, why is SO(3) not ...
Text Solution
|
- Write a balanced chemical equation for the reaction showing catalytic ...
Text Solution
|
- Write the structure of pyrophosphoric acid.
Text Solution
|
- PH(3) forms bubbles when passed slowly in water but NH(3) dissolves. E...
Text Solution
|
- In PCl(5) phosphorus is in sp^(3) d hybridised state but all its five ...
Text Solution
|