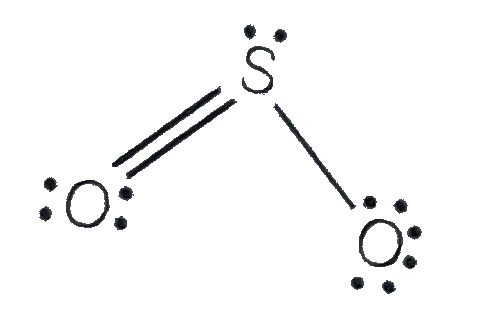
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-P-BLOCK ELEMENTS-P-Block Elements
- Which of the following is correct for P(4) molecule of white phosphoru...
Text Solution
|
- Which of the following statements are correct? I. Among halogens, ra...
Text Solution
|
- Which of the following statements are correct for SO(2) gas? (a) I...
Text Solution
|
- Which of the following statements are correct ? (a) All the three...
Text Solution
|
- Which of the following orders are correct as per the properties mentio...
Text Solution
|
- Which of the following statements are correct? (a) SS bond is prese...
Text Solution
|
- In which of the following reactions conc. H(2)SO(4) is used as an ox...
Text Solution
|
- Which of the following statements are true? (a) Only type of intera...
Text Solution
|
- In the preparation of H(2)SO(4) by Contact process, why is SO(3) not ...
Text Solution
|
- Write a balanced chemical equation for the reaction showing catalytic ...
Text Solution
|
- Write the structure of pyrophosphoric acid.
Text Solution
|
- PH(3) forms bubbles when passed slowly in water but NH(3) dissolves. E...
Text Solution
|
- In PCl(5) phosphorus is in sp^(3) d hybridised state but all its five ...
Text Solution
|
- Why is nitric oxide paramagnetic in gaseous state but the solid obtain...
Text Solution
|
- Give one reason to explain why ClF(3) exists but FCl(3) does not exist...
Text Solution
|
- Out of H(2)O which one has higher bond angle and why?
Text Solution
|
- SF(6) is known but SC(6) is not. Why?
Text Solution
|
- On reaction with Cl(2) phosphorus forms two types of halides 'A' and '...
Text Solution
|
- In the ring test of NO(3)^(-) ion, Fe^(2+) ion reduces nitrate ion to ...
Text Solution
|
- Explain why the stability of oxoacids of chlorine increases in the ord...
Text Solution
|