Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-P-BLOCK ELEMENTS-P-Block Elements
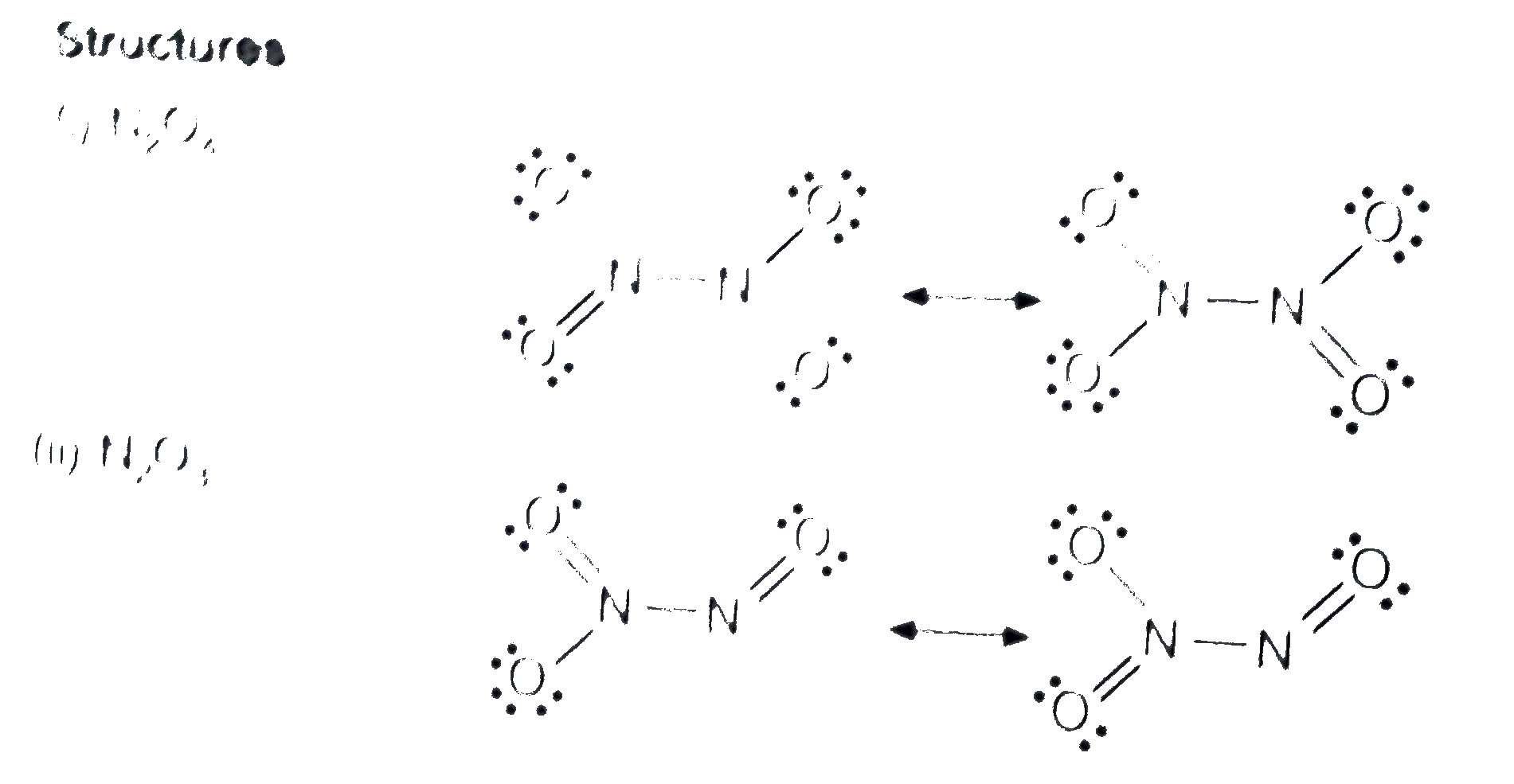
- Name three oxoacids of nitrogen. Write the disproportionation reaction...
Text Solution
|
- Nitric acid forms an oxide of nitrogen on reaction with P(4)O(10). Wri...
Text Solution
|
- (i) White phosphorus (ii) red phosphorus and (iii) black phosphorus. W...
Text Solution
|
- Given an example to show the effect of concentration of nitric acid on...
Text Solution
|
- PCl(5) reacts with finely divided silver on heating and a white silver...
Text Solution
|
- Phosphorus forms a number of oxoacids. Out of these oxoacids, phosphin...
Text Solution
|
- Match the compounds given in Column I with the hybridisation and shape...
Text Solution
|
- Match the formulas of oxides given in Column I with the type of oxide ...
Text Solution
|
- Match the items of Columns I and II and mark the correct option.
Text Solution
|
- Match the species given in Column I with the shape given in Column II ...
Text Solution
|
- Match the items of Columns I and II and mark the correct option.
Text Solution
|
- Assertion (A) N(2) is less reactive than P(4). Reason (R ) Nitrog...
Text Solution
|
- Assertion (A) HNO(3) makes iron passive. Reason (R ) HNO(3) form...
Text Solution
|
- Assertion (A) HI cannot be prepared by the reaction of KI with concent...
Text Solution
|
- Assertion (A) Both rhombic and monoclinic sulphur exist as S(8) but ox...
Text Solution
|
- Assertion (A) NaCl reacts with concentrated H(2)SO(4) to give colourle...
Text Solution
|
- Assertion:- SF(6) cannot be hydrolysed but SF(4) can be. Reason:- Si...
Text Solution
|
- An amorphous solid A burns in air to form a gas B which turns lime w...
Text Solution
|
- On heating lead (II) nitrate gives a brown gas " A". The gas " A" on ...
Text Solution
|
- On heating compound (A) gives a gas (B) which is a constituent of air....
Text Solution
|