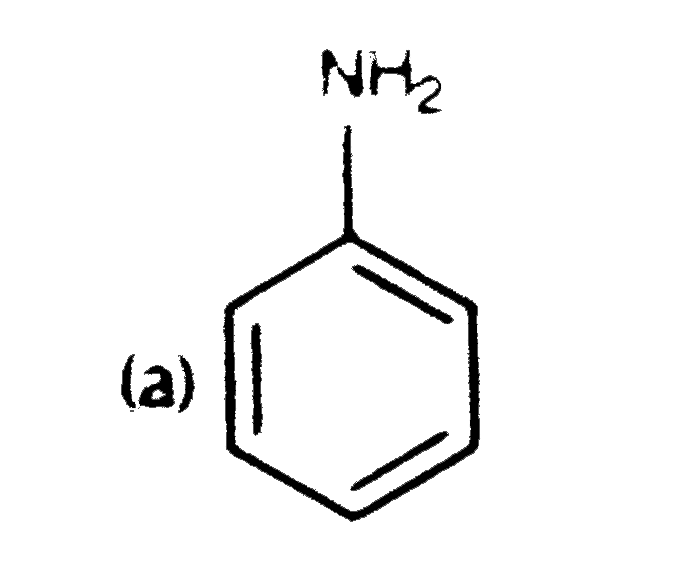
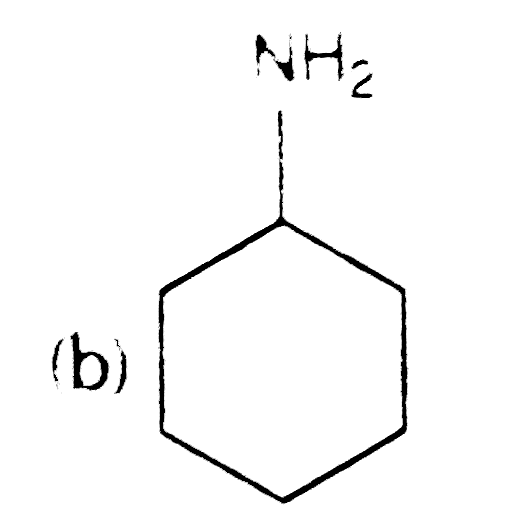
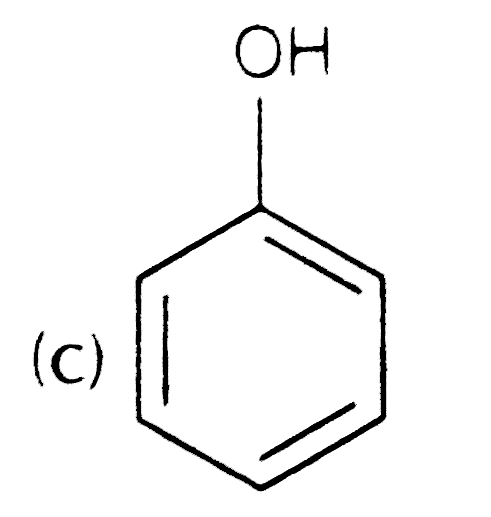
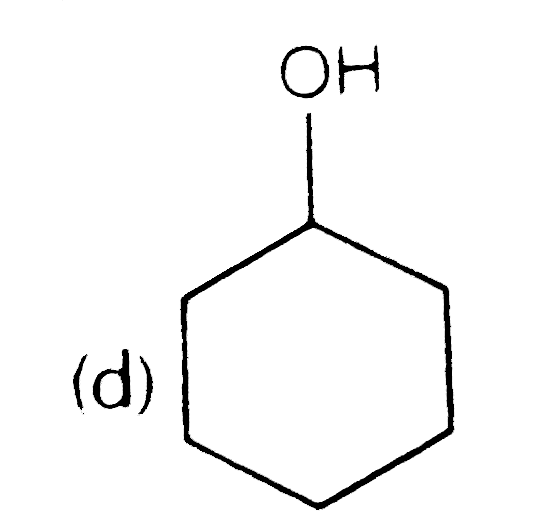
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-AMINES-Amines
- Best method for preparing primary amines from alkyl halides without ch...
Text Solution
|
- Which of the following compounds will not undergo azo coupling reactio...
Text Solution
|
- Which of the following compounds is the weakest Bronsted base?
Text Solution
|
- Among the following amines, the strongest Bronsted base is….
Text Solution
|
- The correct decreasing order of basic strength of the following specie...
Text Solution
|
- Which of the following should be most volatile?
Text Solution
|
- Which of the following methods of preparing of amines will give some n...
Text Solution
|
- Which of the following cannot be prepared by Sandmeyer's reaction?
Text Solution
|
- Reduction of nitrobenzene by which of the following reagent gives anil...
Text Solution
|
- Which of the following species are involved
Text Solution
|
- The reagents that can be used convert benzenediazonium chloride to ben...
Text Solution
|
- The product of the following reaction is …
Text Solution
|
- Arneium ion involved in the bromination of aniline is….
Text Solution
|
- Which of the following can be prepared by Garbriel synthesis?
Text Solution
|
- Which of the following reaction are correct?
Text Solution
|
- Under which of the following reaction condidiotns, aniline give p-nitr...
Text Solution
|
- Which of the following reaction belong to electrophilic aromatic subst...
Text Solution
|
- What is the role of HNO(3) in the nitrating mixture used for nitration...
Text Solution
|
- Why is NH(2) group of aniline acetylated before carrying our nitration
Text Solution
|
- What is the product when C(6)H(5)CH(2)NH(2) reacts with HNO(3)?
Text Solution
|