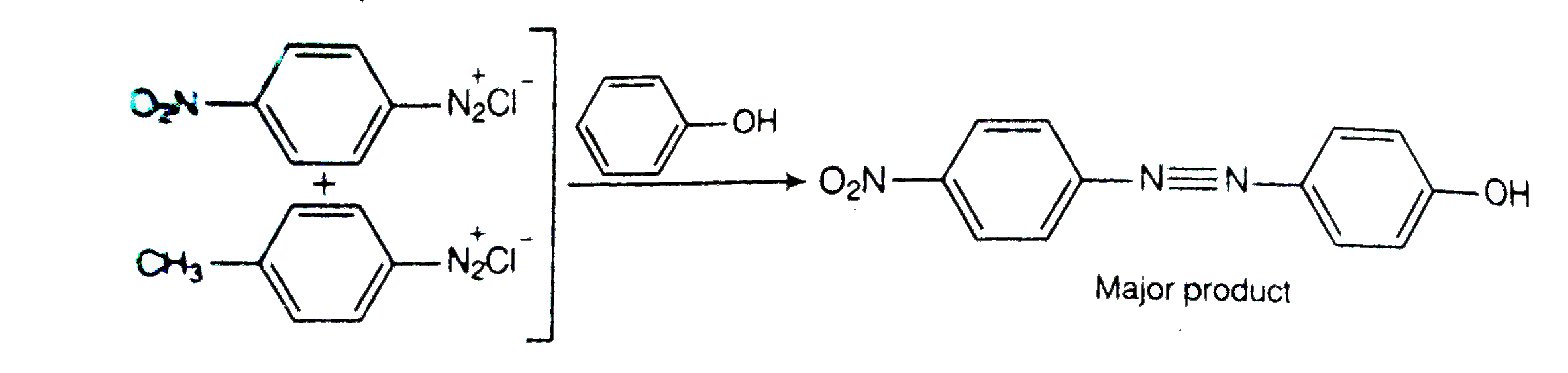Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-AMINES-Amines
- Identfy A and B in the following reactions.
Text Solution
|
- How will you carry out the follwing conversion? (i) Toluene -p-tolui...
Text Solution
|
- Write following conversions (i) Nitrobenzene-Acetanilide (ii) Acet...
Text Solution
|
- A solution contains 1g mol. Each of p-toluene diazonium chloride and p...
Text Solution
|
- How will you bring out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- Match the reactions given in Column I with the statements given in Col...
Text Solution
|
- Match the reactions given in Column I with the statements given in Col...
Text Solution
|
- Assertion(A) Acylation of amines gives a monsubstituted product where...
Text Solution
|
- Assertion (A): Hofmann's bromamide reaction is given by primary amines...
Text Solution
|
- Assertion (A): N-ethylbenzene sulphonamide is solube in alkali. Reas...
Text Solution
|
- Assertion(A): N,N-diethylbenzene sulphonamide is insoluble in alkali. ...
Text Solution
|
- Assertion(A): Only a small amount of HCl is required in the reduction ...
Text Solution
|
- Assertion(A): Aromatic 1^(@ amines can be prepared by Gabriel phtalmi...
Text Solution
|
- Assertion(A): Acetanilide is less basic aniline. Reson(R ): Acetylat...
Text Solution
|
- A hydrocarbon 'A' (C(4)H(8)) on reaction HCl gives a compound 'B', (C(...
Text Solution
|
- A colours substance 'A' (C(6)H(7)) is sparingly soluble in water and g...
Text Solution
|
- Predict the reagent or the product in the following reaction sequence.
Text Solution
|