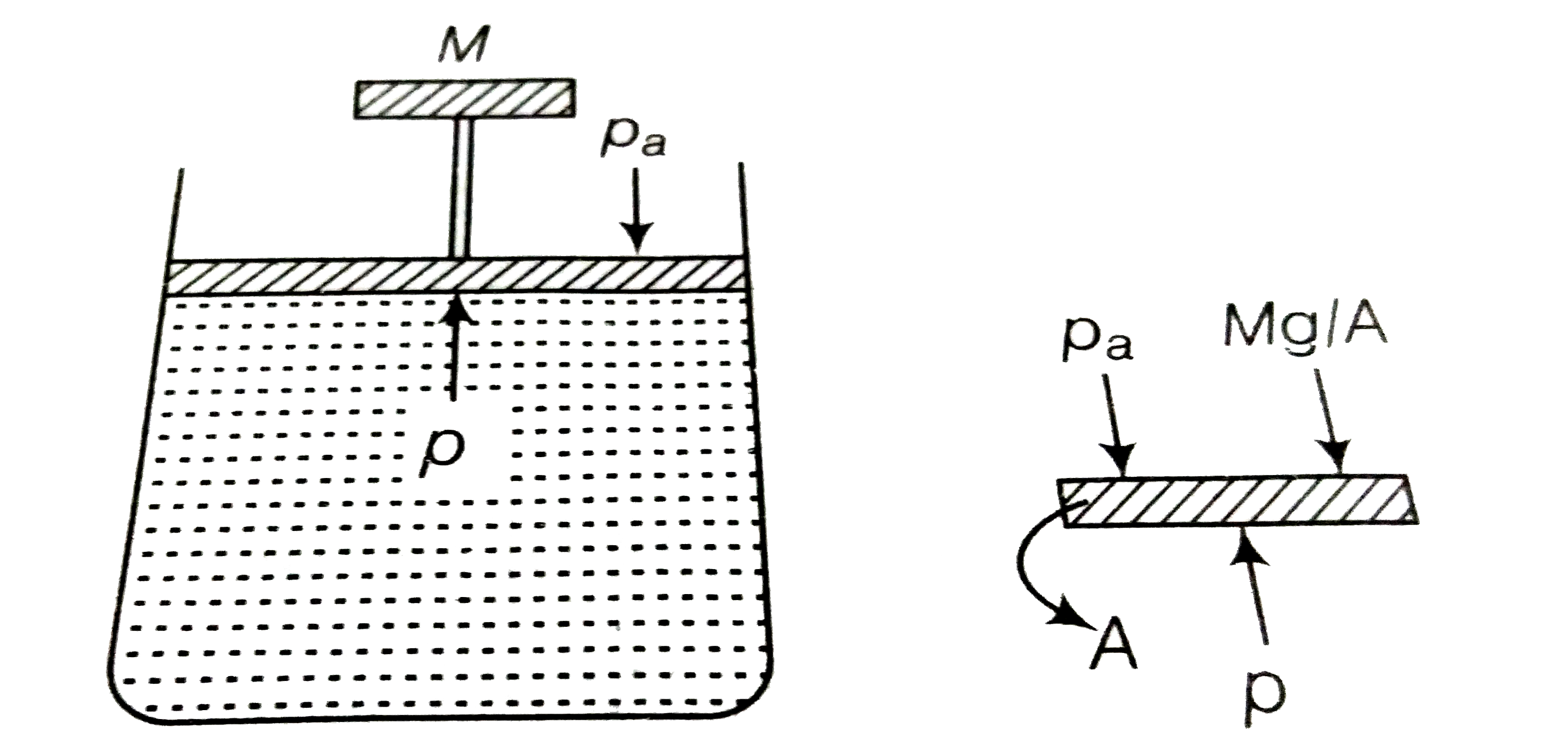
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-KINETIC THEORY-Multiple Choice Questions (MCQs)
- Mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 3...
Text Solution
|
- Boyles's Law is applicable for an
Text Solution
|
- A cylinder containing an ideal gas is in vertical position and has a p...
Text Solution
|
- Volume versus temperature graphs for a given mass of an ideal gas are ...
Text Solution
|
- 1 mole of H(2) gas in contained in a box of volume V 1.00 m^(3) at T =...
Text Solution
|
- A vessel of volume V contains a mixture of 1 mole of hydrogen and 1 mo...
Text Solution
|
- An inflated rubber ballon contains one mole of an ideal gas , has a p...
Text Solution
|
- ABCDEFGH is a hollow cube made of an insulator (figure) face ABCD has...
Text Solution
|
- Diatomic molecules like hydrogen have energies due to both translation...
Text Solution
|
- In a diatiomic molecule, the rotational energy at a given temperature
Text Solution
|
- Which of the following diagrams (figure) depicts ideal gas behaviour?
Text Solution
|
- When an ideal gas is compressed adiabatically , its temperature rises ...
Text Solution
|
- Caculate the number of atoms in 39.4 g gold. Molar mass of gold is 197...
Text Solution
|
- The volume of a given mass of a gas at 27^(@)C, 1 atm is 100cc. What w...
Text Solution
|
- The molecules of a given mass of a gas have root mean square speeds of...
Text Solution
|
- Two molecules of a gas have speeds of 9xx10^(6) ms^(-1) and 1xx10^(6) ...
Text Solution
|
- A gas mixture consists of 2.0 moles of oxygen and 4.0 moles of neon a...
Text Solution
|
- Calculate the ratio of the mean free paths of the molecules of two gas...
Text Solution
|
- The container shown in figure has two chambers separated by a partitio...
Text Solution
|
- A gas mixture consists of molecules of A,B and C with masses m(A) g...
Text Solution
|