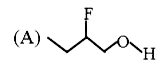
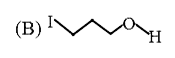
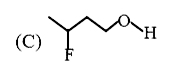
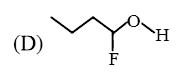
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 1
- Express in decreasing order of (+ I) - (a) CH(3)CH(2) - CH(2) - " ...
Text Solution
|
- Consider the following carbanions (i) CH(3)- overset(Ө)CH(2)" "(ii...
Text Solution
|
- In which of the following compounds is hydroxylic proton the most acid...
Text Solution
|
- Consider following acid underset(I)(ClCH(2)COOH)," "underset(II)(C...
Text Solution
|
- Which of the following acids has lowest pK(a) value?
Text Solution
|
- Arrange in decreasing pK(a) (a) F - CH(2) CH(2) COOH (b)Cl - under...
Text Solution
|
- The correct order of increasing acid strength of the compounds (a) C...
Text Solution
|
- Correct order of basic strength in gas phase is (I) CH(3)-NH(2) (II...
Text Solution
|
- Arrange basicity of the given compounds in decreasing order - (a)CH(...
Text Solution
|
- Which one of the following is the strongest base in aqueous solution?
Text Solution
|
- In which of the following molecules, all atoms are not coplanar ?
Text Solution
|
- (I) CH(2) = CH - CH = CH(2) (II) overset(Theta)CH(2) - CH = CH - ove...
Text Solution
|
- Among these canonical structures, the correct order of stability is
Text Solution
|
- Amongt these canonical structures which one is least stable ?
Text Solution
|
- For phenol which ofthe following resonating structure is the most stab...
Text Solution
|
- The most stable resonating structure of following compound is
Text Solution
|
- Among these canonical structures of pyridiine, the correct order of st...
Text Solution
|
- Write the stability order of Resonating Structures:
Text Solution
|
- ‘M’ effect is the resonance of
Text Solution
|
- Which of the following contain + M but -I effect -
Text Solution
|