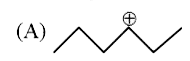
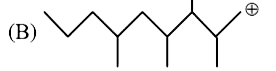
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - I)
- Rank the following free radicals in order of decreasing stability (I...
Text Solution
|
- Rank thefollowing radicals in order of decreasing stability
Text Solution
|
- Select the most stable carboncation among the following -
Text Solution
|
- Writecorrect order of stability of following carbocations :
Text Solution
|
- Arrange the following carbocations in the increasing order of their st...
Text Solution
|
- Which of the following carbocation will be most stable ?
Text Solution
|
- Statement-1: Me - overset(o+)CH(2) is more stable than MeO - CH(2)^(o+...
Text Solution
|
- Ease of ionization to produce carbocation and bromide ion under the tr...
Text Solution
|
- In which of the following pairs, first species is more stable than sec...
Text Solution
|
- The order of stability of the following carbanion is (I) CH(3)overse...
Text Solution
|
- Arrange the carbonions, (CH(3))(3)overset(-)C, overset(-)C Cl(3), (C...
Text Solution
|
- There are three canonical structures of napthalene. Examine them and f...
Text Solution
|
- Which of the following has longest C – O bond:
Text Solution
|
- Among the following molecules, the correct order of C - C bond length ...
Text Solution
|
- In which of the following molecules pi-electron density in ring is max...
Text Solution
|
- Which of these cyclopropene systems is aromatic
Text Solution
|
- Which of these species is anti-aromatic ?
Text Solution
|
- Which of the following compouds is not aromatic
Text Solution
|
- The most stable canonical structure of this molecule is
Text Solution
|
- The most stable canonical structure of this molecule is
Text Solution
|