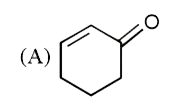
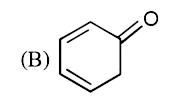
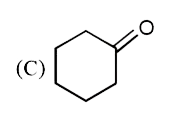
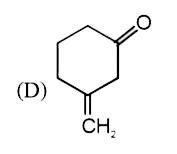
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GOC-Exercise - 2 (Level - I)
- Arrange the carbonions, (CH(3))(3)overset(-)C, overset(-)C Cl(3), (C...
Text Solution
|
- There are three canonical structures of napthalene. Examine them and f...
Text Solution
|
- Which of the following has longest C – O bond:
Text Solution
|
- Among the following molecules, the correct order of C - C bond length ...
Text Solution
|
- In which of the following molecules pi-electron density in ring is max...
Text Solution
|
- Which of these cyclopropene systems is aromatic
Text Solution
|
- Which of these species is anti-aromatic ?
Text Solution
|
- Which of the following compouds is not aromatic
Text Solution
|
- The most stable canonical structure of this molecule is
Text Solution
|
- The most stable canonical structure of this molecule is
Text Solution
|
- The barrier for rotation about the indicated bonds Will be maximum i...
Text Solution
|
- Identify the odd species out Which of the species among the following ...
Text Solution
|
- Which of the following heterocyclic compounds would have aromatic char...
Text Solution
|
- Which one of the following carbonyl compound when treated with dilute ...
Text Solution
|
- The order of the rate of formation of carbocations from the following ...
Text Solution
|
- Write correct order of reactivity of following halogen derivatives tow...
Text Solution
|
- Which of the following species is not aromatic ?
Text Solution
|
- The aromatic character is maximum in which of these three compounds ?
Text Solution
|
- underset((I))(CH(3)COOH)" "underset((II))(CH(3)COONa)" "underset...
Text Solution
|
- Among these compounds, which one has maximum resonance energy ?
Text Solution
|