Text Solution
Verified by Experts
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise QUESTIONS FOR PRACTICE|7 VideosThermodynamics
MOTION|Exercise Solved Example|10 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 3 SECTION b
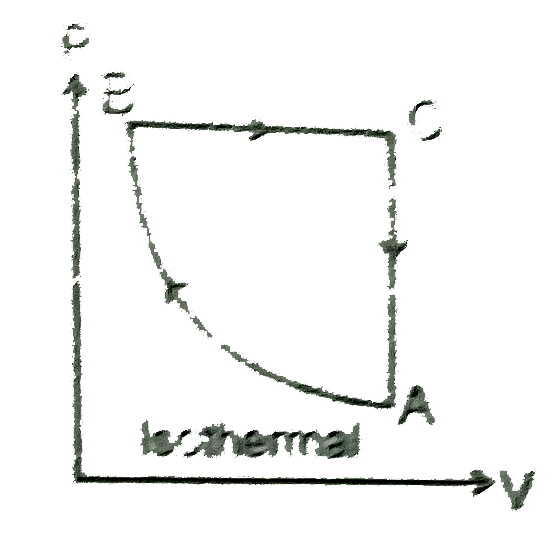
- A cyclic process of an ideal monoatomic gas is shown in figure. The co...
Text Solution
|
- Even Carnot engine cannot give 100% efficiency because we cannot
Text Solution
|
- Two spheres of the same material have radii 1m and 4m and temperatures...
Text Solution
|
- Which statement is incorrect ?
Text Solution
|
- "Heat cannot by itself flow from a body at lower temperature to a body...
Text Solution
|
- Which of the following parameters does not characterize the thermodyna...
Text Solution
|
- A Carnot engine takes 3xx10^6 cal of heat from a reservoir at 627^@C a...
Text Solution
|
- Which of the following statements is correct for any thermodynamic sys...
Text Solution
|
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- A Carnot engine, having an efficiency of eta= 1/10 as heat engine, is ...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- Assume the gas to be ideal, the work done on the gas in taking it from...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- A carnot engine operating between temperatures T(1) and T(2) has effic...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|