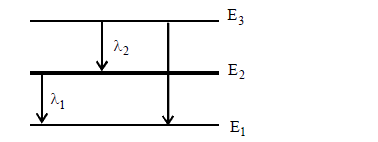Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE & X-RAY -Exercise - 2
- For the given transitions of electron, obtain the relation between la...
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- What is the minimum wavelength of X–rays
Text Solution
|
- What is the approximate ratio of wavelength of radiation K(alpha(1)) a...
Text Solution
|
- To increase the hardness of X–rays in coolidge tube we should –
Text Solution
|
- The solution of Bragg's equation is possible if :
Text Solution
|
- For X–ray diffraction, order of wave length is
Text Solution
|
- Hard X–rays for the study of fractures in bones should have a minimum ...
Text Solution
|
- X-rays were discovered by
Text Solution
|
- Which of the following is the wave length of X– ray:
Text Solution
|
- For producing the X–rays, what is the value of voltage (nearly), which...
Text Solution
|
- For first order X–ray diffraction if lattice constant is 3 xx 10^(–8) ...
Text Solution
|
- lambda("min") of X-rays depends on :-
Text Solution
|
- The order of energy of X-ray photon is :-
Text Solution
|
- If vacuum tube is operated at 6.4 kV, what is the wavelength of X-ray ...
Text Solution
|
- When electron is incident on molyblednum then by changing energy of el...
Text Solution
|
- The groud state energy of hydrogen atom is -13.6 eV. When its electron...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- An electrons of a stationary hydrogen aton passes form the fifth enegr...
Text Solution
|
- Ratio of longest wavelengths corresponding to Lyman and Balmer series ...
Text Solution
|