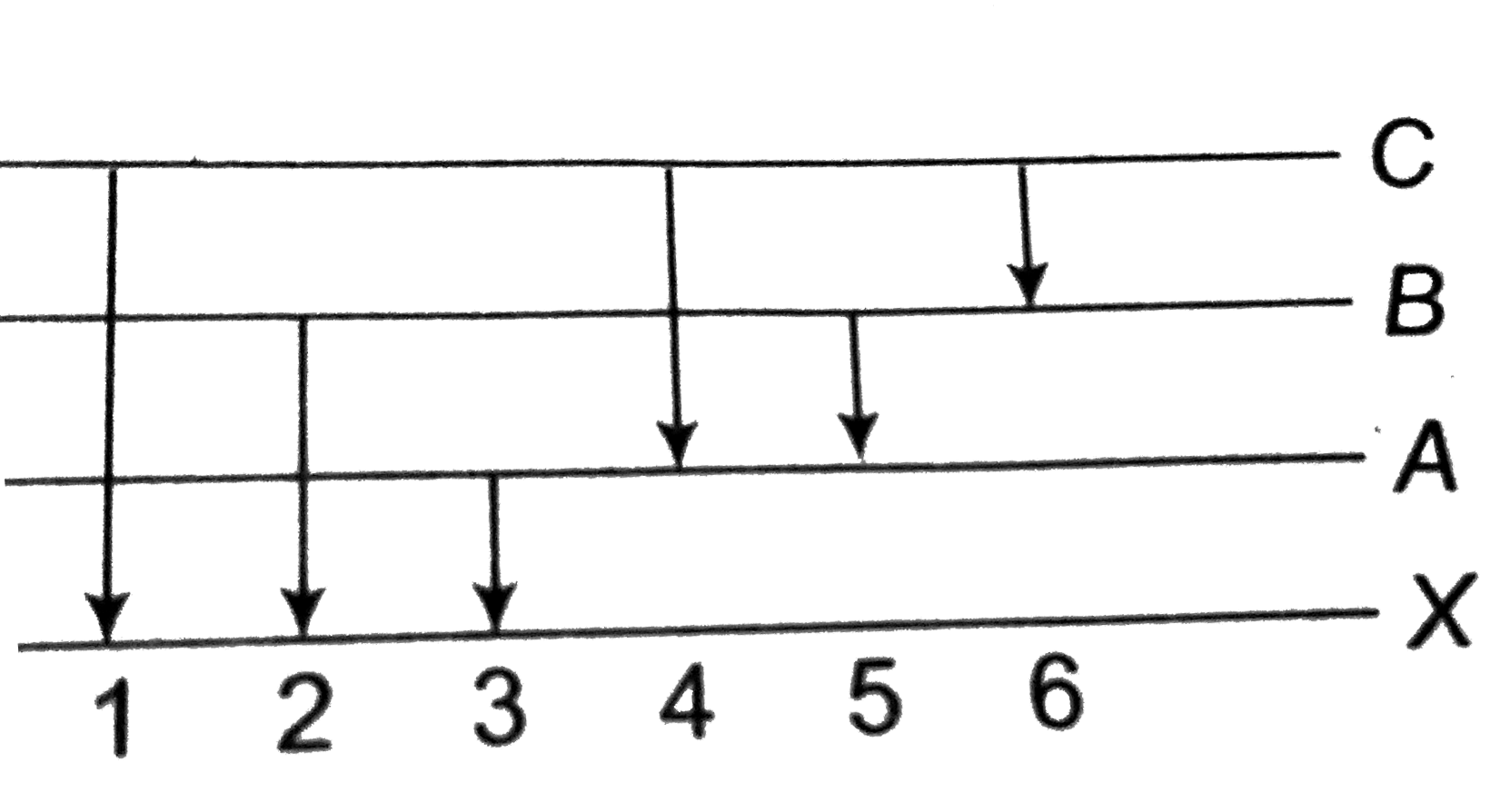
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE & X-RAY -Exercise - 1
- If an electron in hydrogen atom jumps from third orbit to second orbit...
Text Solution
|
- The total energy of an electron in the first excited state of the hydr...
Text Solution
|
- The figure indicates the enegry level diagram of an atom and the origi...
Text Solution
|
- If the energy of a hydrogen atom in nth orbit is E(n), then energy in ...
Text Solution
|
- The ionization potential for hydrogen atom is 13.6 eV, the ionization ...
Text Solution
|
- If the ionization potential of hydrogen atom is 13.6 V, its energy in ...
Text Solution
|
- The ionisation energy of Li^(2+) atom in ground state is,
Text Solution
|
- Which state of the triply ionized Beryllium (Be^(3+)) has the same orb...
Text Solution
|
- What is the wavelength of ligth for the least energetic photon emitted...
Text Solution
|
- What is the ratio of shortest wavelength of the Balmer series ot the ...
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- The series corresponding to minimum wavelength transition in H-atom :-
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- Which series of H(2) atom lie in infrared region?
Text Solution
|
- The Wavelength of first member of Balmer series in hydrogen spectrum i...
Text Solution
|
- Let F1 be the frequency of second line of Lyman series and F2 be the ...
Text Solution
|
- Which of the following may be representing graph between number of sca...
Text Solution
|
- The total energy of eletcron in the ground state of hydrogen atom is -...
Text Solution
|
- In a discharge tube ionization of enclosed gas is produced due to coll...
Text Solution
|