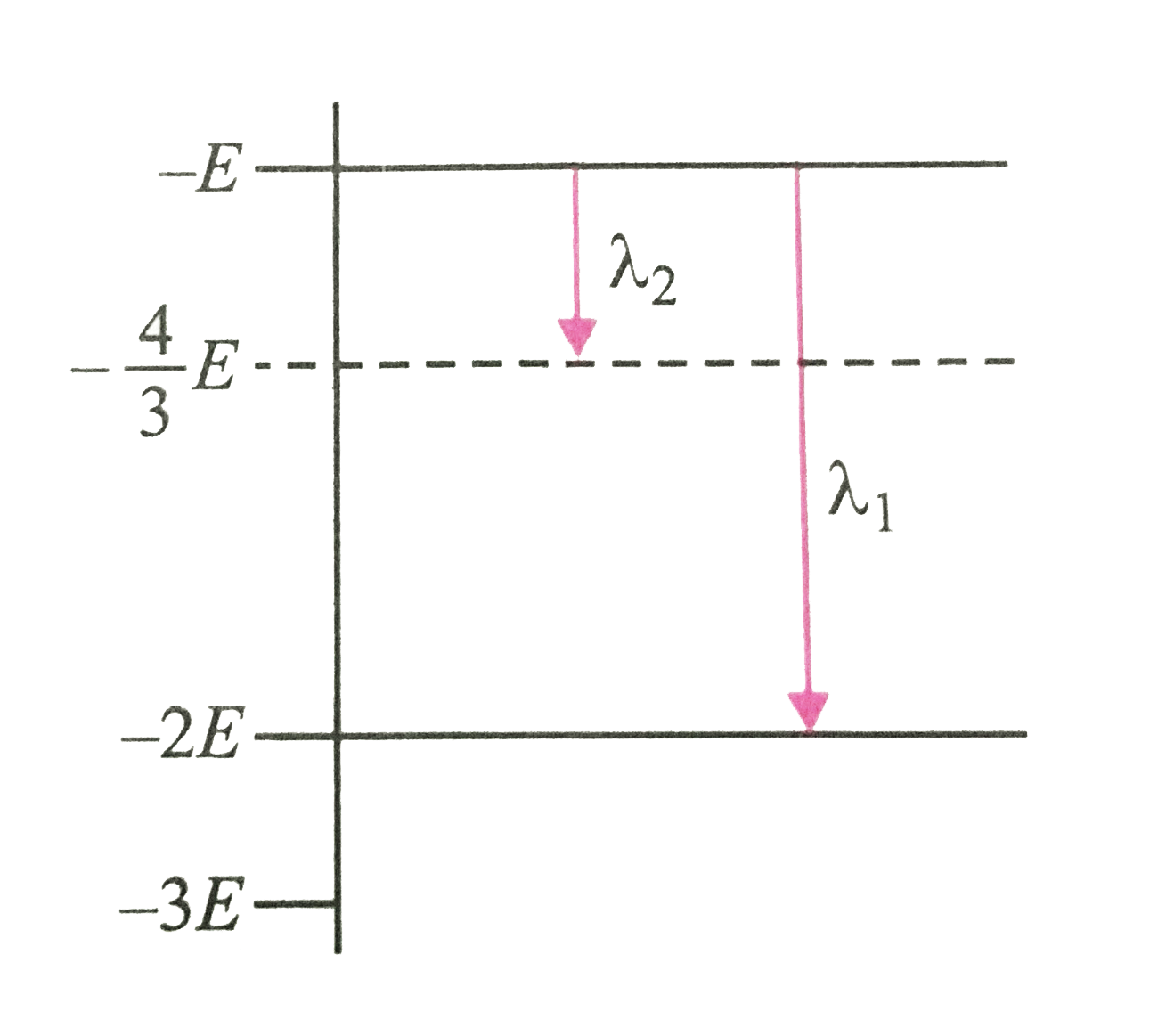
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE & X-RAY -Exercise - 2
- lambda("min") of X-rays depends on :-
Text Solution
|
- The order of energy of X-ray photon is :-
Text Solution
|
- If vacuum tube is operated at 6.4 kV, what is the wavelength of X-ray ...
Text Solution
|
- When electron is incident on molyblednum then by changing energy of el...
Text Solution
|
- The groud state energy of hydrogen atom is -13.6 eV. When its electron...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- An electrons of a stationary hydrogen aton passes form the fifth enegr...
Text Solution
|
- Ratio of longest wavelengths corresponding to Lyman and Balmer series ...
Text Solution
|
- Electrons with de- Broglie wavelength lambda fall on the target in an ...
Text Solution
|
- If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd ...
Text Solution
|
- When an alpha-particle of mass 'm' moving with velocity 'v' bombards o...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- Suppose the charge of a proton and an electron differ slightely. One o...
Text Solution
|
- An electron beam is acceleration by a potential difference V to hit a ...
Text Solution
|
- Some energy levels of a molecule are shown in the fig. The ratio of t...
Text Solution
|
- The ratio of kinetic energy to the total energy of an electron in a Bo...
Text Solution
|
- An electron from various excited states of hydrogen atom emit radiatio...
Text Solution
|
- The mass of hydrogen molecule is 3.32xx10^(-27) kg. If 10^(23) hydroge...
Text Solution
|
- If the series limit frequency of the Lyman series is v(L), then the se...
Text Solution
|