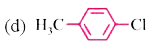
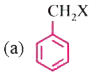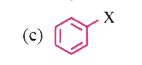A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
CBSE COMPLEMENTARY MATERIAL|Exercise Assertion and Reasoning|2 VideosHALOALKANES AND HALOARENES
CBSE COMPLEMENTARY MATERIAL|Exercise Matching Column Type|2 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
CBSE COMPLEMENTARY MATERIAL|Exercise Short Answer-II Type Questions|5 VideosP-BLOCK ELEMENTS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS|15 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-HALOALKANES AND HALOARENES-CONCEPTUAL QUESTIONS
- Which of the following contain sp^(2) hybridised carbon bonded to X?
Text Solution
|
- Why haloalkanes are more reactive than haloarenes?
Text Solution
|
- Why do haloalkenes under go nucleophillic substitution whereas haloare...
Text Solution
|
- When an alkyl halide is treated with ethanolic solution of KCN, the ma...
Text Solution
|
- The treatment of alkyl chlorides with aqueous KOH leads to the formati...
Text Solution
|
- Explain why vinyl chloride is unreactive in nucleophillic substitution...
Text Solution
|
- Arrange the following compounds according to reactivity towards nucleo...
Text Solution
|
- Why Grignard reagent should be prepared under an hydrous conditions?
Text Solution
|
- Why is Sulphuric acid not used during the reaction of alcohols wiht KI...
Text Solution
|
- p-dichlorobenzene has highest m.p. than those of ortho and m-isomers?
Text Solution
|
- Give reasons: (i) C–Cl bond length in chlorobenzene is shorter than ...
Text Solution
|