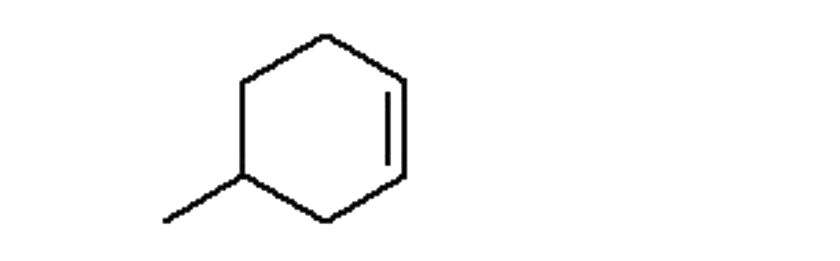
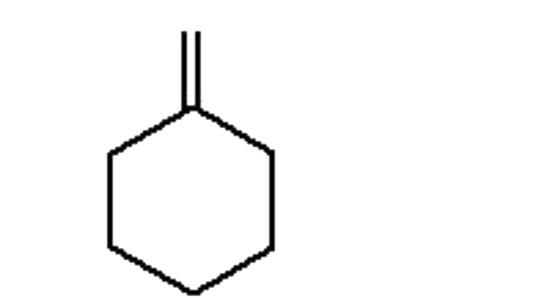
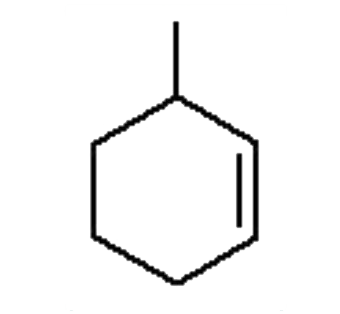
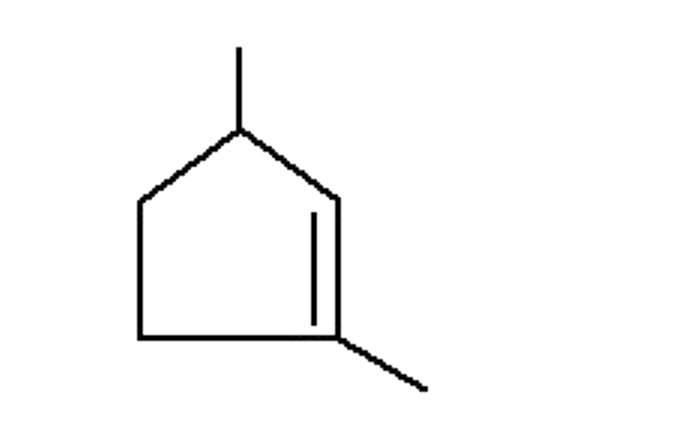
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 28-CHEMISTRY
- Metals which will not evolve H2 gas with dil. HCl are
Text Solution
|
- The reaction X+Y rarrZ is first order with respect to X and second ord...
Text Solution
|
- The product of the following reaction is
Text Solution
|
- The correct order of the ability of the leaving group is
Text Solution
|
- D - Glucose and D - Mannose are :
Text Solution
|
- The most basic nitrogen in the following compound is
Text Solution
|
- 1,3- Pentadiene and 1,-4 - pentadiene are compared with respect to the...
Text Solution
|
- Which of the following on treatment with hot concentrated acidified KM...
Text Solution
|
- Terpen - 4 - ol is an active ingredient in tea tree oil has the follow...
Text Solution
|
- The total work done in the following PV curve is
Text Solution
|
- The antiseptic action of Dettol is due to
Text Solution
|
- The vapour pressure of benzene is 53.3 k P(a) at 60.6^(@) but it falls...
Text Solution
|
- CrO3 dissolves in aqueous NaOH to give
Text Solution
|
- A 50 ml solution of pH=1 is mixed with a 50 ml solution of pH=2. The p...
Text Solution
|
- Oxidation states of the metal in the minerals haematite and magnetite ...
Text Solution
|
- Number of molecules among the following having non - zero dipole momen...
Text Solution
|
- The given compound exists in polar form in which there is a close loop...
Text Solution
|
- The metal M crystallizes in a body cantered lattice with cell edge 40 ...
Text Solution
|
- When the following aldohexose exists in its D-configuration, the total...
Text Solution
|
- The total number of sigma bonds in the structure of P4O10 is
Text Solution
|