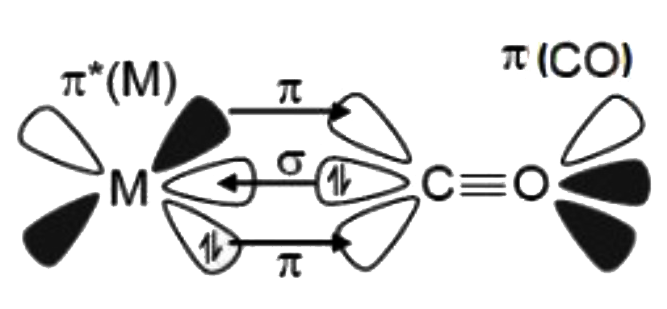
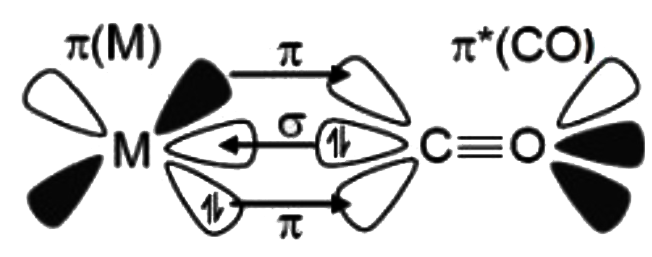
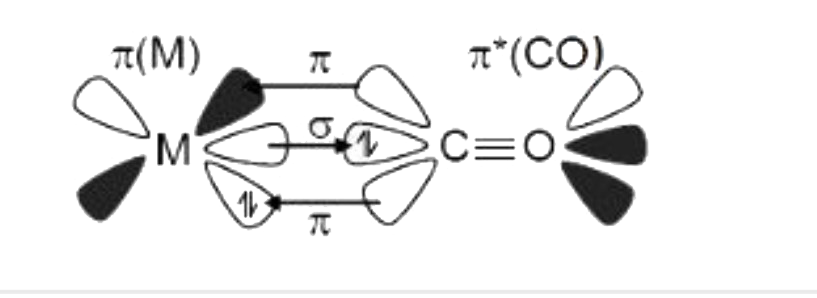
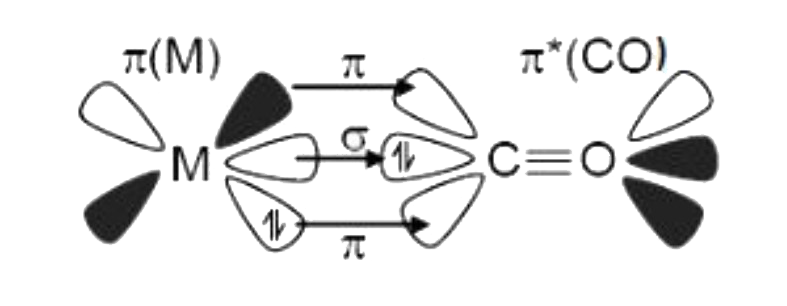
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 30-CHEMISTRY
- In the following reaction sequence the major product B is
Text Solution
|
- Consider the following reaction Which of these reactions are poss...
Text Solution
|
- Metal 'M' forms a carbonly compound in which it is present in its lowe...
Text Solution
|
- Acetic acid (CH(3)COOH) is partially dimerised to (CH(3)COOH(2)) in th...
Text Solution
|
- Which of the following is the correct method for synthesising methyl-t...
Text Solution
|
- In the following reaction, three product a, b, c are obtained. H(3)C...
Text Solution
|
- 100 mL of 0.3 M acetic acid is shaken with 0.8 g wood charcoal. The fi...
Text Solution
|
- The reaction that doses not produce nitrogen is :
Text Solution
|
- For an electron whose positional uncertainly is 1.0xx10^(-10)m, the un...
Text Solution
|
- The standard electrode potentials, E^(@) of Fe^(3+)//Fe^(2+) and Fe^(2...
Text Solution
|
- One mole crystal of a metal halide of the type MX with molecular weigh...
Text Solution
|
- Identify the correct statement :
Text Solution
|
- A reaction is carried out at 600 K. If the same reaction is carried ou...
Text Solution
|
- The ratio of the masses of methane and ethane in a gas mixture is 4:5....
Text Solution
|
- Adsorpton of gases on solid surface is generally exothermic because :
Text Solution
|
- Bleaching action of H(2)O(2) is due to its :
Text Solution
|
- The antibiotic used for curing tuberculosis is :
Text Solution
|
- The maximum number of atoms that may will in the same plane in the fol...
Text Solution
|
- Consider the following reactions : NaCl+K(2)Cr(2)O(7)+underset("(Con...
Text Solution
|
- The number of correctly matched combination is/are {:("Ore","Element...
Text Solution
|